Jérôme Charmet received the Diplôme d’Ingénieur in Microtechnology Engineering from HES-SO Arc in Switzerland in 1998, the M.Sc. degree in Biomedical Engineering from the University of Bern, Switzerland, in 2010, and the PhD degree from the University of Cambridge in 2015. Overall, he worked for more than 10 years in both industrial and academic positions, including Intel Corporation, the National Centre for Sensor Research of Dublin City University in Ireland, the Microtechnology Institute of HES-SO Arc in Switzerland and the Centre for Misfolding Diseases of the University of Cambridge, UK. He joined the University of Warwick as an Assistant Professor in 2016 where he is developing integrated microfluidic platforms to study complex fluids and biological environments with applications in diagnosis, monitoring and drug screening/discovery. Read more about his group research here.
Read his Emerging Investigator article “Resolving protein mixtures using microfluidic diffusional sizing combined with synchrotron radiation circular dichroism” and read about him in the interview below:
Your recent Emerging Investigator Series paper focuses on protein mixtures using microfluidic diffusional sizing combined with synchrotron radiation circular dichroism. How has your research evolved from your first article to this most recent article?
It has evolved quite a lot, in fact it was not even directly related to microfluidics!
What aspect of your work are you most excited about at the moment?
I have started to explore organs-on-a-chip platforms with some colleagues biologists and I find it quite fascinating. But to be honest, every aspects of my work is exciting. I have a great team and collaborators I really enjoy to work with on a daily basis!
In your opinion, what applications can your current approach be used for?
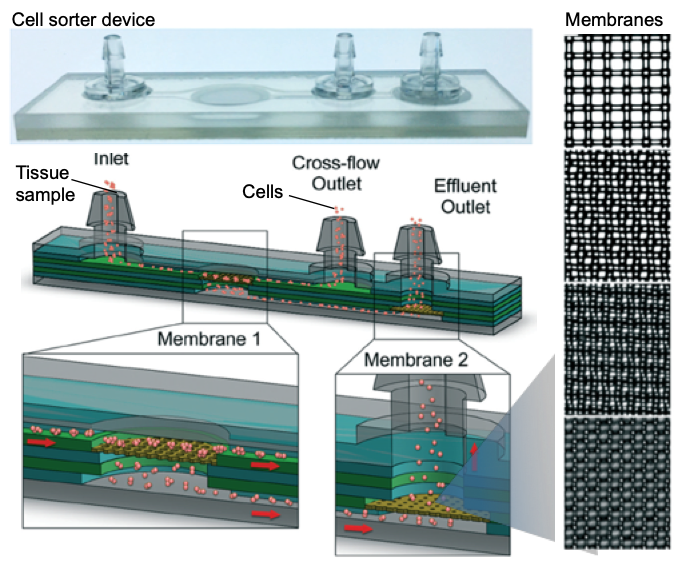
In the manuscript we have used diffusional sizing to resolve the secondary structure of a complex mixture of proteins using synchrotron radiation circular dichroism, but the approach can be applied to other biomolecules with other bulk measurement techniques. We are taking advantage of laminar flow to separate the mixture into “controllable” fractions. By measuring the mixture and the different fractions, we can retrieve information about each component in the mixture.
What do you find most challenging about your research?
It’s multidisciplinary nature.. but it is also one of the most rewarding (when it works).
In which upcoming conferences or events may our readers meet you?
I’m just back from MicroTAS 2018 in Kaohsiung (Taiwan). Next, I will be attending the 8th Annual UK and Ireland Early Career Blood Brain Barrier Symposium 2018 in Oxford.
How do you spend your spare time?
Hiking, running … and these days spending as much time as possible with my 1 year old son.
Which profession would you choose if you were not a scientist?
I got to work with art conservator-restorers (…for some reason) and it is something I would definitely enjoy.
Can you share one piece of career-related advice or wisdom with other early career scientists?
It will sound a bit cheesy, but I will say “believe in your own ideas and importantly, find the right environment to develop them”.