Dr. Aran received her undergraduate degree in electrical engineering at the City University of New York in 2007 and her Ph.D in biomedical engineering at Rutgers University in 2012. She then continued her postdoctoral studies in bioengineering at the University of California Berkeley and was a recipient of National Institutes of Health (NIH) postdoctoral training fellowship at the Buck Institute for Age Research in 2015. She joined Keck Graduate Institute in 2017 as an Assistant Professor and is a currently a visiting scientist at the University of California Berkeley. She is also a consultant for Bill and Melinda Gate Foundation and cofounder of NanoSens innovations. Since starting her faculty position in 2017, Dr. Aran work has been supported by various funds including one RO1, industry sponsored research and a 3 years subaward from University of California Berkeley.
Dr. Aran received her undergraduate degree in electrical engineering at the City University of New York in 2007 and her Ph.D in biomedical engineering at Rutgers University in 2012. She then continued her postdoctoral studies in bioengineering at the University of California Berkeley and was a recipient of National Institutes of Health (NIH) postdoctoral training fellowship at the Buck Institute for Age Research in 2015. She joined Keck Graduate Institute in 2017 as an Assistant Professor and is a currently a visiting scientist at the University of California Berkeley. She is also a consultant for Bill and Melinda Gate Foundation and cofounder of NanoSens innovations. Since starting her faculty position in 2017, Dr. Aran work has been supported by various funds including one RO1, industry sponsored research and a 3 years subaward from University of California Berkeley.
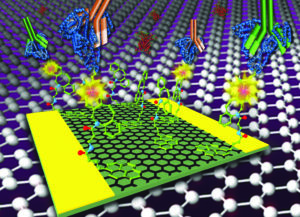
Read her Emerging Investigator Series article “Graphene-based biosensor for on-chip detection of Bio-orthogonally Labeled Proteins to Identify the Circulating Biomarkers of Aging during Heterochronic Parabiosis” and find out more about her in the interview below:
Your recent Emerging Investigator Series paper focuses on digital detection of tagged proteins in vivo to identify the circulating biomarkers in aging. How has your research evolved from your first article to this most recent article?
I was a recipient of an NIH T32 award during my last years of postdoc training at UC Berkeley (2015-2017) which provided me with the opportunity to work with Dr. Irina Conboy, a pioneer in utilizing bio-orthogonally labeled proteins for research on aging. This approach enabled the detection of several rejuvenating protein candidates from the young parabiont, which were transferred to the old mammalian tissue through their shared circulation. Although this method was very powerful, there were a lot of challenges with the detection of these tagged proteins including very time consuming, complex and expensive assays, large sample requirement and false positive. I started working on this project as a side project along with other projects I had with Dr. Conboy just to make the process more facile so we can detect these protein faster and more continuously. This work is a great example of utilizing a lab on a chip technology for a real application. I have also started collaboration with nanomedical diagnostics, a startup company in San Diego, to enable mass production of our chips with high reproducibility. With my academic and industrial collaborators, we are planning to utilize this technology for at least two different applications in the near future to better understand the biology of aging.
What aspect of your work are you most excited about at the moment?
I am very excited to see my technologies go beyond publications. Two of my inventions have been licensed toward developing medical devices for drug delivery and medical diagnostics and the joy from that have been the biggest drive for me to work harder.
In your opinion, what is the future of portable devices for biosensing?
Even though still in its emerging stage, digital diagnostics and 2D materials will shape the future of biosensors.
What do you find most challenging about your research?
As a junior faculty, it is difficult to recruit highly motivated postdoctoral researchers. Unfortunately, the majority of postdoctoral candidates often look for a well-established laboratory headed by a leading scientist. Students and postdoc should know that working in a new lab can be challenging but can provide researcher with opportunities to strengthen their scientific and management skills.
In which upcoming conferences or events may our readers meet you?
I will be presenting some of my biosensor work in the upcoming IEEE EMBS MNMC Conference
How do you spend your spare time?
I love what I do so I can not define my spare time from my work time. I have recently co-founded a startup in digital diagnostics so that takes my weekend but I love it. I also paint when I feel like painting. But I do run, swim and work out routinely after work and enjoy a variety of sports. I have also started traveling to small villages around the world with my friends where we help in building schools.
Which profession would you choose if you were not a scientist?
I love painting and would have explored a career in art.
Can you share one piece of career-related advice or wisdom with other early career scientists?
Have a vision. Sometimes we get lost in small career goals such as publishing but if you have a vision of what you are trying to accomplish you can define your career path, you will start working with the right people who support your vision, you will attend the right conferences and workshops for your career development and you will definitely be successful.