Microparticles were first described in 1967 by Peter Wolf, a physician, as ‘minute particulate materials’ when he investigated the platelet activity in human plasma. They were initially used as drug delivery agents because their size is as small as pollens, which can easily go into the human body. Not long after the great promise of microparticles has been realized, and today we use microparticles in numerous applications including pharmaceuticals, biomedicine, bioengineering, cosmetics, printing, and food science. The widespread use is not a coincidence, they can be synthesized from a multitude of materials, i.e. metal, polymer, gel etc. Especially, polymer microparticles, conferring a great versatility in size, shape, and chemistry, gained more attention in industry. Just like their usage areas, fabrication techniques of microparticles vary a lot. Polymer microparticle production is typically done in two ways, first microfluidics-assisted techniques including droplet-based fabrication, flow-lithography-based fabrication, and microjetting; second other techniques including centrifugation, electrohydrodynamics, and molding. With a focus on microfluidics-assisted techniques, we bring a few remarkable and commercialized studies on high throughput production of spherical-shaped and irregular-shaped microparticles to your attention.
Spherical microparticles
Particle monodispersity has to be compromised for high-througput production when using coaxial microfluidic devices, and both features are highly desired in medical applications and industry. Luckily, as a droplet-based fabrication technique, high-throughput step emulsification of microparticles addresses this fundamental problem. David Weitz’s research group at Harvard University has recently reported a droplet generator microchip with 135 step-emulsifier nozzles that produce monodisperse emulsions of polymers at an exceptional throughput of 10K mL/h (Figure 1a).1 This means, monodisperse microparticle production with this device is thousands times higher than a typical droplet generator microchip with one droplet maker and a throughput of 10 mL/h. The chip is made of PDMS, which is a flexible and inexpensive material. Monodispersity at high flow rates is maintained using microchannels connected through an array of parallelized nozzles (Figure 1b). Microparticles are formed at the step between each nozzle and the continuous-phase channel. The formation can be explained by the Laplace pressure difference developing between the nozzle and the symmetric polymer bulb, resulting in suction of the dispersed phase into the bulb. The growing polymer bulb increases the pressure gradient and a neck forms between the nozzle and the bulb due to depletion of dispersed phase, resulting in release of a droplet. This geometry can produce spherical-shaped microparticles. The production efficiency scales linearly with droplet diameter (Figure 1c). Weitz demonstrated the production of oil microcapsules in water with the envision of standardizing the process by converting the emulsifier into a pipettor tip. Such a technology can replace the existing pipettor technology tools including multi-well and robots, and this replacement can serve for parallelizing and automation of the encapsulation chemi- and bio-assays. This technology has recently been introduced to the market by a Switzerland-based startup company called Microcaps.
As an alternative concept, in-air microfluidics is based on the idea of producing droplets at higher flow rates without using microfluidic channels. In the research groups of Detlef Lohse and Marcel Karperien at University of Twente, microparticles were generated using two nozzles, and one of the nozzles is mounted on a vibrating piezoelectric element (Figure 1d). The breakup of the liquid jet ejected from the first nozzle leads to formation of monodisperse droplets, which hit onto a continuous liquid jet ejected from the second nozzle. After passing ‘the meeting point’, both liquids react with each other to form physically-encapsulated microparticles. This technique provides with hundreds to thousands times faster microparticle production when compared to coaxial microchip setups. Such constructs can be especially beneficial in tissue engineering, where rapid fabrication of multi-scale materials with multiple cell types is an ongoing challenge. This technology has recently been introduced to the market by a Dutch startup company called IamFluidics.
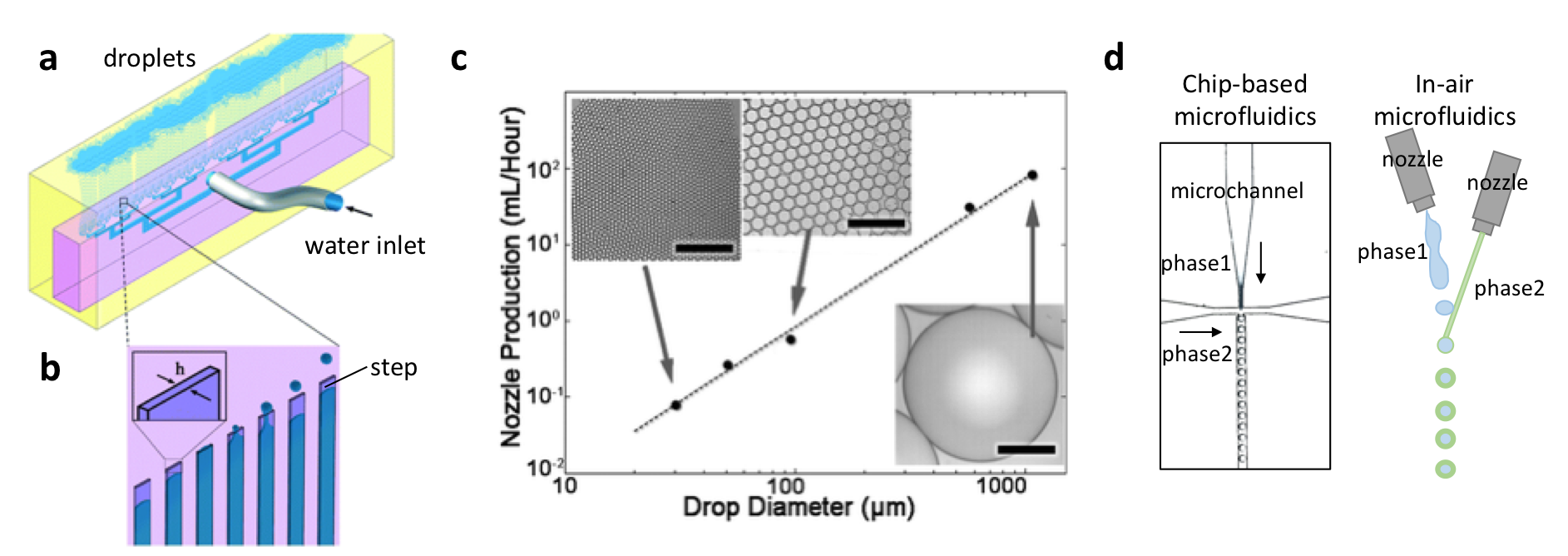
Figure 1. Up-scaled step-emulsification device producing monodisperse droplets. (a) A schematic of the entire microfluidic chip actively producing oil-in-water droplets. (b) The emulsification process. (c) Maximum production rates per nozzle plotted against drop diameter, scale bars are 400 µm.The image is modified from Stolovicki et al. (see the references below). (d) Chip-based microfluidics comparison with in-air microfluidics.
Irregular-shaped microparticles
Another microfluidics-assisted fabrication concept is stop-flow lithography, introduced by Patrick Doyle’s research group at Massachusetts Institute of Technology.2 In this concept, while two (or more) streams of monomers flow side by side through a microchannel made of PFPE coated PDMS, the streams are exposed to intermittent illumination of ultraviolet light through a photomask, which blocks the light selectively. Due to the chemical reaction initiated by ultraviolet light, the liquid solidifies, and forms an individual microparticle (Figure 2a). Upon polymerization, gel particles do not stick to the PFPE microchannel walls, allowing for the production of free-floating particles by the virtue of oxygen lubrication layers. As the ultraviolet light is projected onto the stream through the photomask, each particle takes on the shape of the mask, making the microparticles customizable (Figure 2b). Microparticles composed of multiple monomers can be fabricated by combining multiple monomer streams. The single-step production is advantageous to reduce the production costs, however the particle shape is limited by the photomask and the microchannel geometry – not allowing for generation of spherical-shaped particles. For a proof-of-concept demonstration, upconverting nanocrystal laden-microparticles were synthesized and emitted homogenous visible spectrum of light. The technique allows for synthesis of striped microparticles without losing their homogeneous emission property. The microparticles were also encoded with multiple dot-patterns (Figure 2c), each specific to a target molecule (such as DNA) reacting with the other ingredients in the particle. Such a reaction leads to the formation of a fluorescent color in the microparticle, so the reaction can be traced by microscopy. This technology has been introduced to the market by Firefly Bioworks (acquired by Abcam in 2015), and Motif Micro (acquired by YPB Systems in 2018) startup companies.
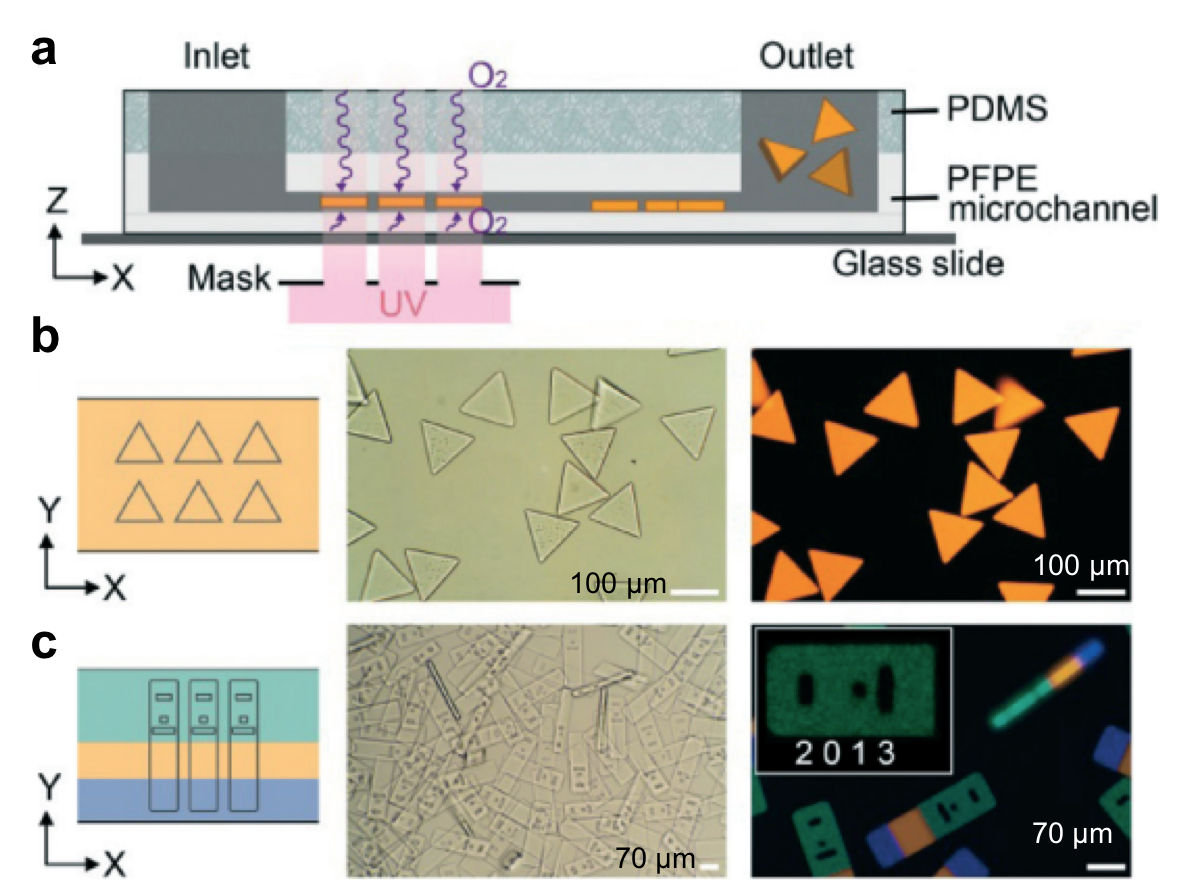
Figure 2. Stop Flow Lithography concept. (a) A schematic demonstration the coaxial microchip. (b) Bright-field and fluorescent images show triangle-shaped particles (c) A mask with an array of barcode particle shapes was aligned on three phase laminar flows in the microchip. Bright-field and fluorescent images show the barcoded particles with three distinct compartments with a region coding “2013”. The image is modified from Bong et al. (see the references below).
To download the full articles click the links below:
1Throughput enhancement of parallel step emulsifier devices by shear-free and efficient nozzle clearance
Elad Stolovicki, Roy Ziblat, and David A. Weitz
Lab Chip, 2018.
DOI: 10.1039/C7LC01037K
2Stop flow lithography in perfluoropolyether (PFPE) microfluidic channels
W. Bong, J. Lee, and P. S. Doyle
Lab Chip, 2014.
DOI: 10.1039/C4LC00877D

About the Webwriter
Burcu Gumuscu is a postdoctoral fellow in Herr Lab at UC Berkeley in the United States. Her research interests include development of microfluidic devices for single-cell analysis, next generation sequencing, compartmentalized organ-on-chip studies, and desalination of water on the microscale.










