Phosphorus is found in natural waters and exerts a major influence on the composition and structure of aquatic ecosystems. It is a crucial nutrient for planktons and algae, which feed fish and other marine organisms. However, human activities may result in excess amount of phosphorus, which, in turn, causes harmful algae to bloom in natural waters. The bloom creates a hostile environment to other forms of marine life by consuming the available oxygen in the sea, and producing toxins. Sea organisms such as fish swim away from the blooms, but the ones that cannot swim, such as shellfish, unfortunately die. We do care about this occurrence as it negatively affects natural life and the economy. There is only one way to interpret the effect of continuously changing phosphorus levels on the strength of the biological pump: real-time monitoring of phosphate levels in the marine environment!
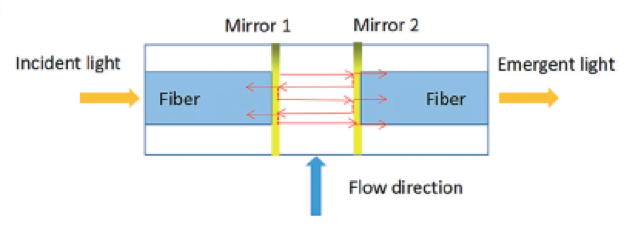
Figure 1. The design of the Fabry-Pérot microcavity, consisting of two parallel mirrors (reflectors) fabricated by coating the surface of the optical fibers with a gold layer. The light is reflected by the mirrors multiple times to enhance the signal. Adapted from Zhu et al., 2017.
Conventional vs. optofluidic monitoring instruments
Conventional phosphate monitoring instruments are mostly used for on-site sampling, then the fresh sample is transported to a laboratory for determining the phosphate level. Laboratories complete one round of analysis in 20 min, often using spectrophotometrical measurement tools. Given the conditions, real-time phosphate monitoring easily becomes laborious, time consuming, and costly. To address this challenge, researchers in Chinese Academy of Sciences, Wuhan University, and The First Institute of Oceanography in China collaborated to develop a portable optofluidic phosphate monitoring tool. However, prototyping an optofluidic marine phosphate detection tool is not straightforward because an absorption cell—a component core to the measurement unit—is simply too big to fit in a microchip. Instead of using a bulky absorption cell, researchers considered integrating a Fabry-Pérot cavity in the microsystem. The Fabry-Pérot cavity consists of two parallel optical fibers with a spacing in between. The cross-sectional surface of each optical fiber is coated with a thin layer of gold to create reflector surfaces (Figure 1) in order to enhance the absorption of phosphate. Shortening the spacing between the reflectors decreases the analysis time from minutes to seconds.
How it works?
In the microchip, filtered water sample and a chromogenic reagent are injected into a curved microchannel. After the chromogenic reaction, the water-soluble components are transported into the optical section (Figure 2). The probe light is sent into the Fabry-Pérot cavity via one of the fibers, bounces between the reflectors multiple times to increase the optical feedback and then analyzed by the detector. The obtained absorbance value, therefore, increases linearly with increasing phosphate concentration. In this microsystem, phosphate detection range is 0.1-100 µmol per liter (400 times greater than the range of a conventional instrument) and detection time is 4 seconds (300 times shorter than detection time of a conventional instrument). The authors of the paper think that this technology can be applied to detect other nutrient levels as well as pH changes in marine environment.

Figure 2. A schematic of the optofluidic microchip consisting of two parts: the microfluidics circuit forming the microreactor in the microchannel, and the optical part to provide optical feedback for enhanced absorption analysis. Adapted from Zhu et al., 2017.
To download the full article for free* click the link below:
Optofluidic marine phosphate detection with enhanced absorption using a Fabry–Pérot resonator
M. Zhu, Y. Shi, X. Q. Zhu, Y. Yang, F. H. Jiang, C. J. Sun, W. H. Zhaoc, and X. T. Hanc
Lab Chip, 2017, Lab on a Chip Recent Hot Articles
DOI: 10.1039/C7LC01016H
Burcu Gumuscu is a postdoctoral fellow in Herr Lab at UC Berkeley in the United States. Her research interests include development of microfluidic devices for quantitative analysis of proteins from single-cells, next generation sequencing, compartmentalized organ-on-chip studies, and desalination of water on the microscale.
*until 16th February 2018











