Like a ripple spreading outwards on a pond’s surface, a plasmon is a collective oscillation of free electrons in a piece of metal. When the metal interacts with light (in this case k vector of light satisfies the momentum condition), the collective movement of oscillating electrons at the metal’s surface leads to the propagation of the light along the surface, also known as a surface plasmon. This simple physics rule is very helpful for sensor nanotechnology and optical signal processing applications since the offered advantages are significant, providing a smaller foot-print, lower limits of detection, and multiplexing opportunities. However, using sensors for biomolecule detection requires clever use of light. For example, surface plasmon resonance can be observed in nanohole arrays patterned on very thin gold films. Nanohole arrays can transmit light much more strongly than expected for the nanohole apertures at certain wavelengths. This phenomenon is called extraordinary optical transmission (EOT) and opens a new avenue for detection of minute amounts of biomolecules.
A nanoplasmonic biosensor was recently developed for real-time monitoring of proteins from live cells in a label-free configuration, thanks to extraordinary optical transmission. The biosensor is structured in a microfluidic device consisting of a cell module and the biosensor module. Microfluidic cell module design is based on a single zigzag channel for directly delivering the secreted proteins to the adjacent biosensor module via a tubing connection. The biosensor module consists of nanoholes fabricated on freestanding SiNx membranes. Antibodies specific to vascular endothelial growth factor (VEGF) are selectively immobilized on the gold nanohole arrays as biorecognition molecules. When the VEGF are captured on the sensor, a change of refractive index proximate to the surface is induced. This change results in a peak shift of the EOT spectrum (Figure 1). By tracing the spectral shifts continuously, the dynamics of cell secretion can be monitored. In this way, the secretion dynamics from live cancer cells are monitored and quantified for 10 hours. The proposed microfluidic device has unique capabilities for multiplexed and label-free detection in a compact footprint, which are promising for miniaturization and integration into lab-on-a-chip devices.
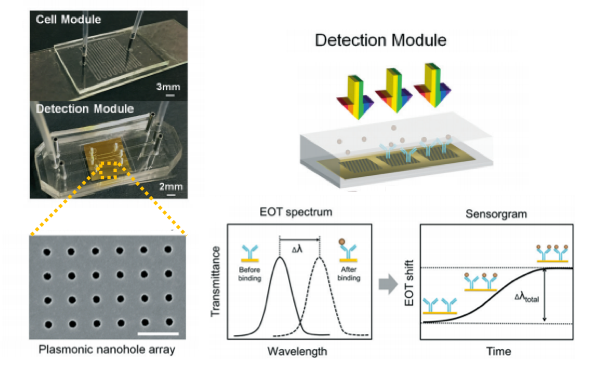
Design and working principle of microfluidic-integrated biosensor. Cancer cells grow in a zigzag channel, and secreted vascular endothelial growth factor are directly delivered to the adjacent detection module. Nanohole structures fabricated in the detection (biosensor) module are shown in SEM image with a scale bar of 1 μm (bottom left). The images on the right show the characteristic resonance peak (solid line) and EOT spectral shift of the peak upon binding (dashed line). Spectral displacements of the resonance peak based on molecular binding accumulation is shown in the sensorgram to reveal the real-time binding dynamics. Adapted from Li et al. (Lab Chip, 2017).
This technology will have a potentially significant impact on medicine. Most of the patients agree that preventive medicine is preferable to reactive medicine. However, preventive medicine requires frequent physical exams, which can only be maintained by spending substantial time and money at physicians’ offices. The need for frequent check-up exams could be reduced or eliminated by real-time monitoring of the health status by portable biosensors in the future. Developing biosensors that allow real-time monitoring of target biomolecules is a big step towards the addressing this future goal.
To download the full article for free* click the link below:
Plasmonic nanohole array biosensor for label-free and real-time analysis of live cell secretion
Xiaokang Li, Maria Soler, Cenk I. Özdemir, Alexander Belushkin, Filiz Yesilköy and Hatice Altug
Lab Chip, 2017
DOI: 10.1039/C7LC00277G
*Free to access until 7th August 2017.
Burcu Gumuscu is a postdoctoral fellow in Herr Lab at UC Berkeley in the United States. Her research interests include development of microfluidic devices for quantitative analysis of proteins from single-cells, next generation sequencing, compartmentalized organ-on-chip studies, and desalination of water on the microscale.











