Multiplexing slanted spiral microchannels for ultra-fast blood plasma separation
Lab on a Stick: Multi-Analyte Cellular Assays in a Microfluidic Dipstick
Highly Efficient Adenoviral Transduction of Pancreatic Islets using a Microfluidic Device
Introducing graphene into microfluidic devices can make it easier to study proteins at an atomic level, scientists in the US have shown. Devices that are thinner and interfere less with the measurements allow larger and more intricate protein structures to be resolved using techniques that rely on probing thousands of microcrystals.
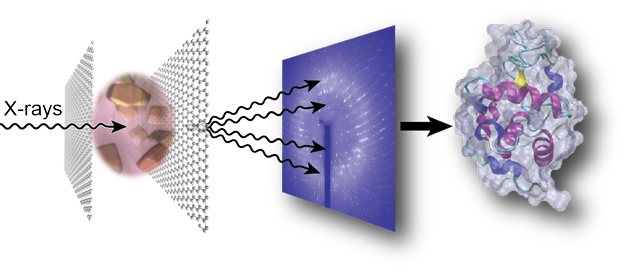
Not only does this reduce the device’s thickness, improving the signal-to-noise ratio, the graphene also acts as a barrier to prevent the sample evaporating. John Helliwell, an expert in crystallography at the University of Manchester in the UK, explains that preventing water loss from the crystal is ‘vital…because the sample hydration state needs to be preserved for its molecular integrity’.
Perry’s group are now focusing on shrinking down the dimensions and increasing the complexity of the device, as well as studying the structure of proteins involved in programmed cell death.
To read the full article visit Chemistry World.
Click the link below to read the original research paper published in Lab on a Chip for free*:
Graphene-based microfluidics for serial crystallography
Shuo Sui, Yuxi Wang, Kristopher W. Kolewe, Vukica Srajer, Robert Henning, Jessica D. Schiffman, Christos Dimitrakopoulos and Sarah L. Perry
Lab Chip, 2016, Advance Article
DOI: 10.1039/C6LC00451B
*Article is free to access until 26/07/2016 through a RSC registered account – click here to register
Lance Munn and co-workers at Massachusetts General Hospital have developed tissue isolation chambers that can be implanted into the brain or skin of mice beneath a transparent window, to allow host-tumour interactions to be observed over a timescale of weeks to months. A small tumour fragment from a donor mouse was placed within the shallow ‘tumour isolation chamber’ and implanted into another mouse, forcing any vasculature and connective tissue (stroma) to occur in an essentially 2D space. Fluorescent reporters were then used to visualise specific components of the tissue.

Different implantable tissue isolation chambers that were developed. a) 'raft' model; b) 'hole' model; c) 'pillar' model; d) transparent window models in the dorsal skin or brain
By using a shallow chamber, this new method overcomes some of the limitations of other systems used for studying tumour microenvironments and stromal remodelling processes. Other in vivo mouse models use fluorescent reporter and even transparent windows, however the penetration depth of optical microscopy is only a few hundred micrometers, preventing observations below this depth in the tissue. By using tissue chambers of around 150 µm, this issue is circumvented. Another problem can be in visualising structures that extend in the z direction, as they may overlap and be hidden; this is overcome in this work by allowing freedom of movement in the x-y plane, while restricting movement or growth in the z direction.
In the studies carried out by the authors, tumour angiogenesis was clearly observed and was found to show the same properties usually observed in tumours. It was also found that migrating blood vessel sprouts were closely associated with bundles of collagen fibres, providing the first evidence for matrix-guided sprouting in tumour angiogenesis. The tissue isolation chambers also allowed analysis of processes that are difficult to study through other methods, due to either the short distances involved, low frequency of occurrence, or rapid dynamics.
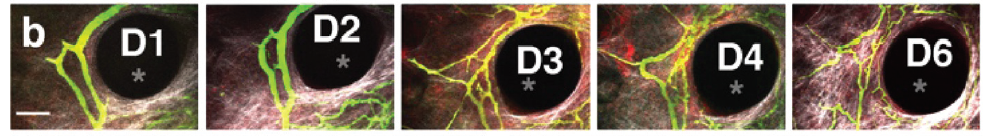
Image sequence showing the expansion of vessel sprouts and vascular loops in the tumour isolation chamber. D1=day 1, etc. Pillar structure is indicated by an asterix.
One potential application of this technology highlighted by the authors, is to provide vascularised tissues for transplantation, allowing good blood supply to the transplanted tissue immediately after implantation. In the experiments reported in this paper, stable and mature vasculature was formed that remained functional for more than 2 months after the tissue chambers were implanted. Although these initial findings are very positive, further studies would need to be carried out on a wide range of tissue types.
To download the full article for free* click the link below:
Implantable tissue isolation chambers for analyzing tumor dynamics in vivo
Gabriel Gruionu, Despina Bazou, Nir Maimon, Mara Onita-Lenco, Lucian G. Gruionu, Peigen Huang and Lance L. Munn
Lab Chip, 2016,16, 1840-1851
DOI: 10.1039/C6LC00237D
—————-

About the webwriter
Claire Weston is a PhD student in the Fuchter Group, at Imperial College London. Her work is focused on developing novel photoswitches and photoswitchable inhibitors.
—————-
*Access is free until 30/06/2016 through a registered RSC account – click here to register