Shefali Lathwal and Hadley Sikes at Massachusetts Institute of Technology have carried out an in-depth study published in Lab on a Chip comparing different colorimetric paper-based immunoassays (where a positive or negative result is shown by the appearance or absence of a certain colour). The assays used were all for the detection of an enzyme found in P. falciparum in order to diagnose malaria. The authors sought to identify the optimal readout times for the different methods in order to prevent false positives.
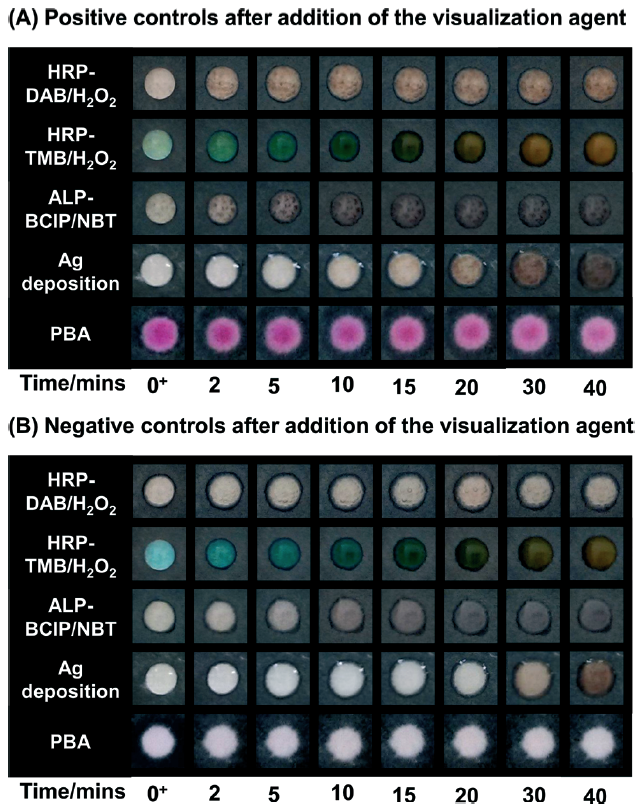
Time course for colour generation on negative and positive surfaces using different colorimetric methods
One of the main purposes of this study was to compare a new paper-based assay that had recently been developed by Sikes in collaboration with George Whitesides at Harvard (Lab on a Chip, 2015) with other state-of-the-art methods.
The new method in question is a colorimetric assay that utilises a photo-initiated polymerisation reaction to amplify the signal when the P. falciparum enzyme is present. The reaction only occurs when the sample is being irradiated, so by using an automated timing switch the reaction time can be accurately controlled and no further signal amplification will take place once the light has been turned off. In contrast, other methods require accurate manual time keeping as they are thermally rather than photochemically controlled. This means that if the sample is left too long, it may lead to false positives due to colour forming in a negative sample.
This can be seen in the figure on the right, where positive and negative controls detected using the different colorimetric methods were photographed at various time points. All the methods tested had the same binding events in order to allow a fair comparison (i.e., all assay steps were the same apart from the detection method). A diagram included in the manuscript (Scheme 2) shows the key steps to all the assays, and how the colour is formed.
Three of the methods were enzymatic amplifications; for these reactions t=0 was taken as when the substrate solution was added to the surface of the paper. Another method was silver deposition, and t=0 was taken as when the silver enhancement solution was added to the surface. For the polymerisation-based amplification (PBA) method, the aqueous monomer was added to the surface and after illumination a basic solution was added, which led to formation of colour in the positive samples; t=0 was taken as when the basic solution was added.
In all cases other than the photo-controlled reaction, colour developed in the negative controls over time, leading to very similar results as in the positive controls. These assays are usually used at the point of care in resource limited settings, therefore the readouts are carried out by eye and there is often not a negative control to compare to. Instead, the result is compared to a colour chart, making it even easier to obtain a false positive if the readout time is not correct.
For the enzymatic amplification and silver deposition the optimal readout times varied considerably and in some cases the time window was very narrow, in order to prevent false positives. In the PBA reaction however, no colour developed in the negative control over 40 minutes, and at all time intervals there was a clear difference between the positive and negative controls. In addition to this, the visual limit of detection for the PBA reaction was much higher than that of the enzymatic amplifications and silver deposition.
This study is the first to compare multiple colorimetric methods for paper-based immunoassays with carefully controlled variables. Previously, different binding reagents, imaging techniques and methods of quantification have meant that meaningful comparisons could not be obtained. The results clearly highlight the benefits of using a photo-controlled reaction, where the reaction time can be carefully controlled with an automated timer without the requirement of accurate manual time keeping.
To download the full article for free* click the link below:
Assessment of colorimetric amplification methods in a paper-based immunoassay for diagnosis of malaria
Shefali Lathwal and Hadley D. Sikes
Lab Chip, 2016, Advance Article
DOI: 10.1039/C6LC00058D
—————-

About the webwriter
Claire Weston is a PhD student in the Fuchter Group, at Imperial College London. Her work is focused on developing novel photoswitches and photoswitchable inhibitors.
—————-
*Access is free until 27/04/2016 through a registered RSC account – click here to register










