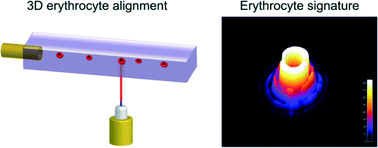
Flow cytometry is used to examine blood cells, which requires hydrodynamic sheath flow alignment and fluorescence antibody labelling, making it time-consuming and expensive. Advanced light scattering techniques (such as digital holography) are often seen as suitable alternatives, as they provide fast and label-free measurements.
In a recent Lab On A Chip article, Netti et al. from the Italian Institute of Technology, in collaboration with scientists from Germany and Russia, presented a camera-based light scattering approach, coupled with a viscoelasticity -induced cell migration technique. This new system is used to characterise the morphological properties of erythrocytes in microfluidic flows.
They obtained light scattering profiles (LSPs) of individual living cells in microfluidic flows over a wide angular range and matched them with scattering simulations to characterise their morphological properties. A healthy erythrocyte diameter lies between 6 and 9 µm. The diameter values obtained from the experiment lie between 7 and 8.3 µm, which is in good agreement with the existing literature.
‘The results demonstrate the ability of a rapid and cost effective way to measure the average dimensions of an erythrocyte population which can be easily related to the health of a patient,’ concludes Netti.
To gain deeper insight into LSP acquisition and simulation, you can read the full article for free* by following the link below.
Optical signature of erythrocytes by light scattering in microfluidic flows
D. Dannhauser, D. Rossi, F. Causa, P. Memmolo, A. Finizio, T. Wriedt, J. Hellmers, Y. Eremin, P. Ferraro and P. A. Netti
Lab Chip, 2015,15, 3278-3285
DOI: 10.1039/C5LC00525F