Over the years, the materials used to make microfluidic devices have dictated the progress of the field. The development of early silicon and glass devices progressed very slowly because the fabrication methods required to make these devices were prohibitively expensive and inaccessible.1 Since the arrival of polydimethylsiloxane (PDMS)-based devices made by elastomeric micromolding or “soft lithography” in 1998,2 the pace of microfluidic technology development has increased dramatically. For example, between 1998 and 2010, the number of microfluidic-related publications increased from hundreds to thousands per year.3 These developments were fueled by the simplicity of PDMS-soft lithography, and more importantly, the ability of PDMS to form pneumatic valves and pumps.4
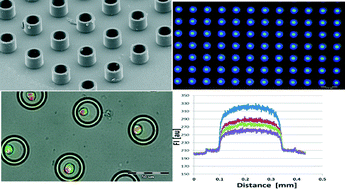
Although soft lithography has become one of the most popular methods for microfluidic fabrication, clean room processes are still needed to make a micromold, and PDMS is not compatible with existing high-throughput manufacturing methods.1 For these reasons and others, researchers are developing alternative methods for device fabrication. For example, Dr. Cooksey at the National Institute of Standards and Technology, Gaithersburg and Prof. Atencia at the University of Maryland developed techniques to create microfluidic devices from cut-off laminates and double-sided tapes.5 Their article, which featured as a cover in Lab on a Chip, showed how these film-based devices can be rapidly fabricated without a cleanroom using in-expensive materials and widely available equipment (e.g. razor cutter, laser cutter).

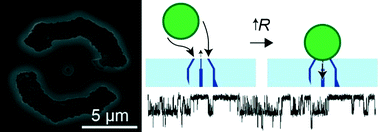
Like conventional PDMS-devices, these film-based devices can support pneumatic valves and pumps by sandwiching a thin layer of PDMS between two layers of film with cut-out channels. Accurate alignment between these layers is achieved using a self-alignment strategy, in which features of adjacent layers are mirrored across a folding line on a single piece of tape. To demonstrate valve functionality, Cooksey and Atencia created a device that uses 3 valves to control flow from 3 fluidic inputs, and one that uses 8 valves to control a 2-inlet rotary mixer.
One interesting feature of this technology is that very thin devices can be formed (less than 0.5 mm), which enables the fabrication of devices with many layers. For example, using the self-alignment strategy, the researchers fabricated a 6-layer device comprising a valve layer and five fluidic layers that form liquid chambers of varying heights.
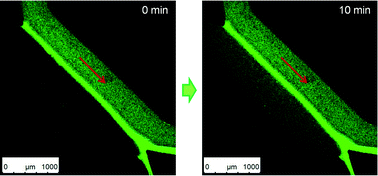
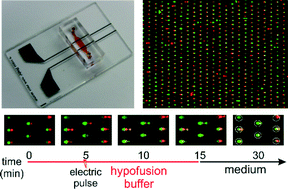
Perhaps the most fascinating trait of this technology is the ability to fold devices into 3D structures with fully functioning valves. As shown in the figure below, the researchers assembled a 3D microfluidic cube that can deliver reagents to specific locations on the cube using the fluid channels routed through the walls. The researchers filled the cube with agar and used it to study the chemotaxis of C. elegans. Within two hours, the worms migrated from the center of the cube toward the face introduced with food, and promptly moved away when the food was switched to a repellent.
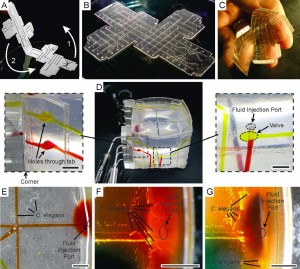
In summary, Dr. Cooksey and Prof. Atencia developed a rapid prototyping technique that can create film-based devices with the similar valve functionalities as conventional PDMS-based devices. But because these devices are very thin, more complicated and unique devices structures can be created. This technology has the potential uncover new applications for microfluidics, and make microfluidic technologies more accessible to non-engineers (e.g. biologists and clinicians).
1. E. K. Sackmann, A. L. Fulton and D. J. Beebe, Nature, 2014, 507, 181-189.
2. D. C. Duffy, J. C. McDonald, O. J. A. Schueller and G. M. Whitesides, Analytical Chemistry, 1998, 70, 4974-4984.
3. E. Berthier, E. W. K. Young and D. Beebe, Lab on a Chip, 2012, 12, 1224-1237.
4. M. A. Unger, H.-P. Chou, T. Thorsen, A. Scherer and S. R. Quake, Science, 2000, 288, 113-116.
5. Pneumatic valves in folded 2D and 3D fluidic devices made from plastic films and tapes, Gregory A. Cooksey and Javier Atencia, Lab on a Chip, 2-14, 14, 1665-1668