Researchers at the City University of Hong Kong and Jinan University, China, have developed a rapid approach to study how cells “feel” the forces around them.
Cells sense and react to their physical surroundings through mechanotransduction—the translation of mechanical stimulation into biochemical signals. These signals, in turn, regulate many important physiological processes such as those in the immune system, bone metabolism and blood circulation. Since defects in mechanotransduction are associated with various human diseases,1 understanding their molecular basis may aid in the development of new therapeutics. Unfortunately, most techniques for studying mechanotransduction are slow and labour-intensive, requiring meticulous manipulation of single cells for mechanical stimulation and analysis.
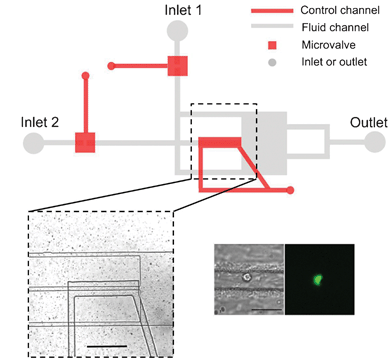 To address this challenge, a research team led by Prof. Mengsu Yang has developed a new microfluidic chip that can isolate individual cells and apply precise whole-cell mechanical stimulation.
To address this challenge, a research team led by Prof. Mengsu Yang has developed a new microfluidic chip that can isolate individual cells and apply precise whole-cell mechanical stimulation.
The microfluidic chip is assembled by sandwiching a deflectable polymer membrane between a fluid layer and a control layer. The chip comprises three parallel channels, two microvalves and one compressive component in the middle channel (see picture). By modulating the pressure in the control channels, the height of the middle channel can be adjusted to trap or compress an individual cell from a suspension. The microvalves can be opened or closed to exchange the reagent environment surrounding the trapped cell.
Using this chip, the research team studied the intracellular calcium signaling in HL60 cells (leukemic cells) triggered by whole-cell compression. They demonstrate that mechanical compression can activate ion channels in the cell membrane, causing extracellular calcium ions to transport inside the cell. Interestingly, they show that an intact cytoskeleton was not required for the activation of mechanosensitive ion channels in HL60 cells—the involvement of the cytoskeleton in mechnotranduction remains a much-debated topic in the field.
Taken together, the research team successfully demonstrated that this microfluidic chip is a fast, useful platform for the study of mechanotransduction in individual suspension cells. In the future, the throughput of this chip may be extended by parallelizing several compressive components on one chip.
1. Jaalouk, D. E.; Lammerding, J. Nature Reviews Molecular Cell Biology 2009, 10, 63-73.
Microfluidics study of intracellular calcium response to mechanical stimulation on single suspension cells
Tao Xu, Wanqing Yue, Cheuk-Wing Li, Xinsheng Yao and Mengsu Yang
DOI: 10.1039/C3LC40880A
Alphonsus Ng is a PhD student in the Wheeler Microfluidics Laboratory, University of Toronto, Canada










