View the new videos on the Lab on a Chip YouTube site below:
.
.
.
A single-channel microparticle sieve based on Brownian ratchets
View the new videos on the Lab on a Chip YouTube site below:
.
.
.
A single-channel microparticle sieve based on Brownian ratchets
 The Pioneers of Miniaturisation Lecture is awarded annually to an early to mid-career scientist for extraordinary or outstanding contributions to the understanding or development of miniaturised systems. This year’s presentation of the award will take place during the µTAS 2012 Conference in Okinawa, Japan, in October 2012. The Lectureship is jointly awarded by the Lab on a Chip and Corning Incorporated and includes $5000 ($2000 of which may be used to attend the µTAS Symposium).
The Pioneers of Miniaturisation Lecture is awarded annually to an early to mid-career scientist for extraordinary or outstanding contributions to the understanding or development of miniaturised systems. This year’s presentation of the award will take place during the µTAS 2012 Conference in Okinawa, Japan, in October 2012. The Lectureship is jointly awarded by the Lab on a Chip and Corning Incorporated and includes $5000 ($2000 of which may be used to attend the µTAS Symposium).
Nominations are now invited for this award – the deadline for nominations is 28th May 2012. Full details of the criteria and how to submit the nominations are to be found on the Pioneers of Miniaturisation Lecture webpage.

Micro-immunohistochemistry using a microfluidic probe
Robert D. Lovchik, Govind V. Kaigala, Marios Georgiadis and Emmanuel Delamarche
DOI: 10.1039/C2LC21016A

A self-loading microfluidic device for determining the minimum inhibitory concentration of antibiotics
Nate J. Cira, Jack Y. Ho, Megan E. Dueck and Douglas B. Weibel
DOI: 10.1039/C2LC20887C
The issue also includes the Focus article ‘Standards for collecting microfluidic devices?‘ by Henne van Heeren at enablingMNT, discussing the need for standards for microfluidic interconnections, chip dimensions and a vocabulary, the latest article in our Acoustofluidics series on the acoustic radiation force on small particles by Henrik Bruus at the Technical University of Denmark, and the following HOT articles:
Sorting cells by size, shape and deformability
Jason P. Beech, Stefan H. Holm, Karl Adolfsson and Jonas O. Tegenfeldt
DOI: 10.1039/C2LC21083E
A microfluidic method to study demulsification kinetics
Thomas Krebs, Karin Schroen and Remko Boom
DOI: 10.1039/C2LC20930F
Design considerations for electrostatic microvalves with applications in poly(dimethylsiloxane)-based microfluidics
Amit V. Desai, Joshua D. Tice, Christopher A. Apblett and Paul J. A. Kenis
DOI: 10.1039/C2LC21133E
All our HOT articles are free to access for four weeks (following a simple registration for individual users).
This month sees the following articles in Lab on a Chip that are in the top ten most accessed:
Bubbles no more: in-plane trapping and removal of bubbles in microfluidic devices
Conrad Lochovsky, Sanjesh Yasotharan and Axel Günther
Lab Chip, 2012, 12, 595-601
DOI: 10.1039/C1LC20817A
Rapid, multiplexed microfluidic phage display
Kellye Cung, Russell L. Slater, Yue Cui, Sharon E. Jones, Habib Ahmad, Rajesh R. Naik and Michael C. McAlpine
Lab Chip, 2012, 12, 562-565
DOI: 10.1039/C2LC21129G
Design of pressure-driven microfluidic networks using electric circuit analogy
Kwang W. Oh, Kangsun Lee, Byungwook Ahn and Edward P. Furlani
Lab Chip, 2012, 12, 515-545
DOI: 10.1039/C2LC20799K
Microfluidic capture and release of bacteria in a conical nanopore array
Peng Guo, Eric W. Hall, Romana Schirhagl, Hitomi Mukaibo, Charles R. Martin and Richard N. Zare
Lab Chip, 2012, 12, 558-561
DOI: 10.1039/C2LC21092D
Droplet-based microfluidic device for multiple-droplet clustering
Jing Xu, Byungwook Ahn, Hun Lee, Linfeng Xu, Kangsun Lee, Rajagopal Panchapakesan and Kwang W. Oh
Lab Chip, 2012, 12, 725-730
DOI: 10.1039/C2LC20883K
A digital microfluidic method for multiplexed cell-based apoptosis assays
Dario Bogojevic, M. Dean Chamberlain, Irena Barbulovic-Nad and Aaron R. Wheeler
Lab Chip, 2012, 12, 627-634
DOI: 10.1039/C2LC20893H
Fish and Chips: a microfluidic perfusion platform for monitoring zebrafish development
Deepak Choudhury, Danny van Noort, Ciprian Iliescu, Baixue Zheng, Kar-Lai Poon, Svetlana Korzh, Vladimir Korzh and Hanry Yu
Lab Chip, 2012, 12, 892-900
DOI: 10.1039/C1LC20351G
A microchamber array for single cell isolation and analysis of intracellular biomolecules
Klaus Eyer, Phillip Kuhn, Conni Hanke and Petra S. Dittrich
Lab Chip, 2012, 12, 765-772
DOI: 10.1039/C2LC20876H
Photolithographic surface micromachining of polydimethylsiloxane (PDMS)
Weiqiang Chen, Raymond H. W. Lam and Jianping Fu
Lab Chip, 2012, 12, 391-395
DOI: 10.1039/C1LC20721K
Microfluidic approach for highly efficient synthesis of heparin-based bioconjugates for drug delivery
Thanh Huyen Tran, Chi Thanh Nguyen, Dong-Pyo Kim, Yong-kyu Lee and Kang Moo Huh
Lab Chip, 2012, 12, 589-594
DOI: 10.1039/C1LC20769E
Why not take a look at the articles today and blog your thoughts and comments below.
Fancy submitting an article to Lab on a Chip? Then why not submit to us today or alternatively email us your suggestions.
View the new videos on the Lab on a Chip YouTube site below:
.
Cell sorting by deterministic cell rolling
.
.
.
Sorting cells by size, shape and deformability
.
Nebulisation on a disposable array structured with phononic lattices
.
.
.
An on-chip whole blood/plasma separator using hetero-packed beads at the inlet of a microchannel
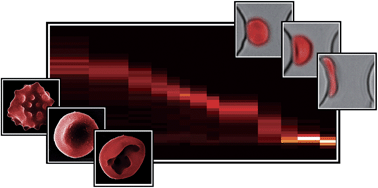
Sorting cells by size, shape and deformability
Jason P. Beech, Stefan H. Holm, Karl Adolfsson and Jonas O. Tegenfeldt
DOI: 10.1039/C2LC21083E
Thomas Krebs and colleagues at Wageningen University present the results of experiments studying droplet coalescence in a dense layer of emulsion droplets using microfluidic circuits:
A microfluidic method to study demulsification kinetics
Thomas Krebs, Karin Schroen and Remko Boom
DOI: 10.1039/C2LC20930F
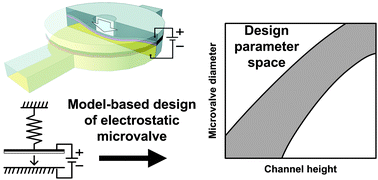
Design considerations for electrostatic microvalves with applications in poly(dimethylsiloxane)-based microfluidics
Amit V. Desai, Joshua D. Tice, Christopher A. Apblett and Paul J. A. Kenis
DOI: 10.1039/C2LC21133E
David Beebe et al. discuss the use of PDMS and polystyrene by researchers working at the interface of microfluidics and cell biology research:
Engineers are from PDMS-land, Biologists are from Polystyrenia
Erwin Berthier, Edmond W. K. Young and David Beebe
DOI: 10.1039/C2LC20982A
These HOT articles are free to access for the next four weeks (following a simple registration for individual users), so why not take a look?
Scientists in Canada have developed a method to study the changes in red blood cells caused by the most common malaria parasite Plasmodium falciparum.
The malaria parasite is spread by mosquitos. © Shutterstock
Malaria causes approximately one million deaths each year and there is a lot of research surrounding the disease’s biomechanics, aimed at diagnosing patients and developing treatments. The parasite, spread by mosquitoes, infects red blood cells and reduces their ability to deform. Measuring the deformability of an infected red blood cell can provide vital information about the disease’s mechanism and response to treatment.
Current methods to measure deformability are complicated or not sensitive enough, but Hongshen Ma at the University of British Columbia, Vancouver, and colleagues, have designed an accurate and simple microfluidic device for this purpose. The device consists of two layers that control the cells so that only a single cell is introduced into a funnel containing a series of different sized constrictions. The pressure required to push the cell through a constriction is measured precisely and used to calculate the deformability.
The team used their device to show that the deformability of uninfected red blood cells can be distinguished from cells at various stages of infection.
Abhishek Jain at Boston University, US, is an expert in biomedical devices and comments that ‘the device is elegant in the sense that it can be easily scaled up for diagnosis and high throughput drug testing for not only malaria but other pathologies like sickle cell anaemia’.
‘We hope our technique will provide a useful biomechanical assay for the development of new drugs,’ says Ma, who adds that ‘the ability to easily measure the deformability of red blood cells will help researchers study the mechanism of the disease and investigate complex challenges, such as drug resistance.’
Ma’s team plans to further test their device and use it to study the mechanisms of drug resistance.
Microfluidic biomechanical assay for red blood cells parasitized by Plasmodium falciparum
Quan Guo , Sarah J. Reiling , Petra Rohrbach and Hongshen Ma
Lab Chip, 2012, Advance Article
DOI: 10.1039/C2LC20857A
Original article published at Chemistry World

Batch fabrication of disposable screen printed SERS arrays
Lu-Lu Qu, Da-Wei Li, Jin-Qun Xue, Wen-Lei Zhai, John S. Fossey and Yi-Tao Long
DOI: 10.1039/C2LC20926H
On the inside front cover is another hot article, this time from Andrew Griffiths et al. who have developed an ultrahigh-throughput system which combines droplet PCR and IVTT to provide a completely in vitro screen.
A completely in vitro ultrahigh-throughput droplet-based microfluidic screening system for protein engineering and directed evolution
Ali Fallah-Araghi, Jean-Christophe Baret, Michael Ryckelynck and Andrew D. Griffiths
DOI: 10.1039/C2LC21035E
Plus you can read the latest Research Highlights from Ali Khademhosseini – shrink-film for cell culture, optically adjustable microfluidic chips and protein profiling with microgels – or the latest in our Acoustofluidics series on measurement techniques for the characterization of ultrasonic particle manipulation devices.
The issue also features several hot articles which will be free to access* for 4 weeks:
Rapid, sensitive, and multiplexed on-chip optical sensors for micro-gas chromatography
Karthik Reddy, Yunbo Guo, Jing Liu, Wonsuk Lee, Maung Kyaw Khaing Oo and Xudong Fan
DOI: 10.1039/C2LC20922E
Microfluidic single-cell cultivation chip with controllable immobilization and selective release of yeast cells
Zhen Zhu, Olivier Frey, Diana Silvia Ottoz, Fabian Rudolf and Andreas Hierlemann
DOI: 10.1039/C2LC20911J
Dual-electrode microfluidic cell for characterizing electrocatalysts
Ioana Dumitrescu, David F. Yancey and Richard M. Crooks
DOI: 10.1039/C2LC21181E
* Following a simple registration for individual users
During 2011 we published a number of topical reviews on a wide range of topics by expert researchers in their fields. We’ve collected some of them below but take a look here for the whole list, we hope you’ll find something interesting in your area.
Miles Padgett and Roberto Di Leonardo
Lab-on-a-chip based immunosensor principles and technologies for the detection of cardiac biomarkers: a review
Mazher-Iqbal Mohammed and Marc P. Y. Desmulliez
Nanomanipulation using near field photonics
David Erickson, Xavier Serey, Yih-Fan Chen and Sudeep Mandal
Microfluidics for food, agriculture and biosystems industries
Suresh Neethirajan, Isao Kobayashi, Mitsutoshi Nakajima, Dan Wu, Saravanan Nandagopal and Francis Lin
Hyundoo Hwang and Je-Kyun Park
Disposable microfluidic substrates: Transitioning from the research laboratory into the clinic
Jason S. Kuo and Daniel T. Chiu
Miniaturized isothermal nucleic acid amplification, a review
Peter J. Asiello and Antje J. Baeumner
If you have an idea for a review article that hasn’t been covered and you would like to see included, contact the Editorial Office – we’d love to hear from you.

Fully automated cellular-resolution vertebrate screening platform with parallel animal processing
Tsung-Yao Chang, Carlos Pardo-Martin, Amin Allalou, Carolina Wählby and Mehmet Fatih Yanik
DOI: 10.1039/C1LC20849G

Microfabricated passive vapor preconcentrator/injector designed for microscale gas chromatography
Jung Hwan Seo, Sun Kyu Kim, Edward T. Zellers and Katsuo Kurabayashi
DOI: 10.1039/C2LC20932B
The issue also features a whole host of hot articles on topics from education to droplets, which will be free to access for 4 weeks:
Education: a microfluidic platform for university-level analytical chemistry laboratories
Jesse Greener, Ethan Tumarkin, Michael Debono, Andrew P. Dicks and Eugenia Kumacheva
DOI: 10.1039/C2LC20951A
Self-propelling surfactant droplets in chemically-confined microfluidics – cargo transport, drop-splitting and trajectory control
David K. N. Sinz and Anton A. Darhuber
DOI: 10.1039/C2LC21082G
Droplet-based microfluidic device for multiple-droplet clustering
Jing Xu, Byungwook Ahn, Hun Lee, Linfeng Xu, Kangsun Lee, Rajagopal Panchapakesan and Kwang W. Oh
DOI: 10.1039/C2LC20883K
Quick genotyping detection of HBV by giant magnetoresistive biochip combined with PCR and line probe assay
Xiao Zhi, Qingsheng Liu, Xin Zhang, Yixia Zhang, Jie Feng and Daxiang Cui
DOI: 10.1039/C2LC20949G
Rapid prototyping of three-dimensional microfluidic mixers in glass by femtosecond laser direct writing
Yang Liao, Jiangxin Song, En Li, Yong Luo, Yinglong Shen, Danping Chen, Ya Cheng, Zhizhan Xu, Koji Sugioka and Katsumi Midorikawa
DOI: 10.1039/C2LC21015K
Ultrahigh sensitivity assays for human cardiac troponin I using TiO2 nanotube arrays
Piyush Kar, Archana Pandey, John J. Greer and Karthik Shankar
DOI: 10.1039/C2LC20892J
Also, take a look at the latest article in our acoustofluidics series – Building microfluidic acoustic resonators.