The Editors at Lab on a Chip have been busy picking out the top 10% from all our high quality papers to bring you a collection of recent articles that we think will be of exceptional significance for the miniaturisation community.
Papers in this category will have received excellent reports during peer review, and demonstrate a breakthrough in device technology, methodology or demonstrate important new results for chemistry, physics, biology or bioengineering enabled by miniaturisation.
Here are the papers that have caught our eye so far:
Frontier
Microengineered physiological biomimicry: Organs-on-Chips
Dongeun Huh, Yu-suke Torisawa, Geraldine A. Hamilton, Hyun Jung Kim and Donald E. Ingber
DOI: 10.1039/C2LC40089H
Focus
Education: a microfluidic platform for university-level analytical chemistry laboratories
Jesse Greener, Ethan Tumarkin, Michael Debono, Andrew P. Dicks and Eugenia Kumacheva
DOI: 10.1039/C2LC20951A
Tutorial Review
Brain slice on a chip: opportunities and challenges of applying microfluidic technology to intact tissues
Yu Huang, Justin C. Williams and Stephen M. Johnson
DOI: 10.1039/C2LC21142D
Critical Review
Engineers are from PDMS-land, Biologists are from Polystyrenia
Erwin Berthier, Edmond W. K. Young and David Beebe
DOI: 10.1039/C2LC20982A
Communications
“Fluidic batteries” as low-cost sources of power in paper-based microfluidic devices
Nicole K. Thom, Kimy Yeung, Marley B. Pillion and Scott T. Phillips
DOI: 10.1039/C2LC40126F
Sorting cells by size, shape and deformability
Jason P. Beech, Stefan H. Holm, Karl Adolfsson and Jonas O. Tegenfeldt
DOI: 10.1039/C2LC21083E
Papers
High throughput automated chromatin immunoprecipitation as a platform for drug screening and antibody validation
Angela R. Wu, Tiara L.A. Kawahara, Nicole A. Rapicavoli, Jan van Riggelen, Emelyn H. Shroff, Liwen Xu, Dean W. Felsher, Howard Y. Chang and Stephen R. Quake
DOI: 10.1039/C2LC21290K
A microfluidic device for whole-animal drug screening using electrophysiological measures in the nematode C. elegans
Shawn R. Lockery, S. Elizabeth Hulme, William M. Roberts, Kristin J. Robinson, Anna Laromaine, Theodore H. Lindsay, George M. Whitesides and Janis C. Weeks
DOI: 10.1039/C2LC00001F
Ion diode logics for pH control
Erik O. Gabrielsson, Klas Tybrandt and Magnus Berggren
DOI: 10.1039/C2LC40093F
Human gut-on-a-chip inhabited by microbial flora that experiences intestinal peristalsis-like motions and flow
Hyun Jung Kim, Dongeun Huh, Geraldine Hamilton and Donald E. Ingber
DOI: 10.1039/C2LC40074J
A multifunctional pipette
Alar Ainla, Gavin D. M. Jeffries, Ralf Brune, Owe Orwar and Aldo Jesorka
DOI: 10.1039/C2LC20906C
Visualization of microscale particle focusing in diluted and whole blood using particle trajectory analysis
Eugene J. Lim, Thomas J. Ober, Jon F. Edd, Gareth H. McKinley and Mehmet Toner
DOI: 10.1039/C2LC21100A
DNA electrophoresis in a nanofence array
Sung-Gyu Park, Daniel W. Olson and Kevin D. Dorfman
DOI: 10.1039/C2LC00016D
Bipolar electrochemistry for cargo-lifting in fluid channels
Gabriel Loget and Alexander Kuhn
DOI: 10.1039/C2LC21301J
Rapid screening of antibiotic toxicity in an automated microdroplet system
Krzysztof Churski, Tomasz S. Kaminski, Slawomir Jakiela, Wojciech Kamysz, Wioletta Baranska-Rybak, Douglas B. Weibel and Piotr Garstecki
DOI: 10.1039/C2LC21284F
Dual-electrode microfluidic cell for characterizing electrocatalysts
Ioana Dumitrescu, David F. Yancey and Richard M. Crooks
DOI: 10.1039/C2LC21181E
Rapid, sensitive, and multiplexed on-chip optical sensors for micro-gas chromatography
Karthik Reddy, Yunbo Guo, Jing Liu, Wonsuk Lee, Maung Kyaw Khaing Oo and Xudong Fan
DOI: 10.1039/C2LC20922E
A silicone-based stretchable micropost array membrane for monitoring live-cell subcellular cytoskeletal response
Jennifer M. Mann, Raymond H. W. Lam, Shinuo Weng, Yubing Sun and Jianping Fu
DOI: 10.1039/C2LC20896B
Batch fabrication of disposable screen printed SERS arrays
Lu-Lu Qu, Da-Wei Li, Jin-Qun Xue, Wen-Lei Zhai, John S. Fossey and Yi-Tao Long
DOI: 10.1039/C2LC20926H
Bubbles no more: in-plane trapping and removal of bubbles in microfluidic devices
Conrad Lochovsky, Sanjesh Yasotharan and Axel Günther
DOI: 10.1039/C1LC20817A
A digital microfluidic method for multiplexed cell-based apoptosis assays
Dario Bogojevic, M. Dean Chamberlain, Irena Barbulovic-Nad and Aaron R. Wheeler
DOI: 10.1039/C2LC20893H
Technical Innovation
Three-dimensional microfiber devices that mimic physiological environments to probe cell mechanics and signaling
Warren C. Ruder, Erica D. Pratt, Sasha Bakhru, Metin Sitti, Stefan Zappe, Chao-Min Cheng, James F. Antaki and Philip R. LeDuc
DOI: 10.1039/C2LC21117C
We will be adding to this collection throughout the year so keep checking back for more outstanding articles!
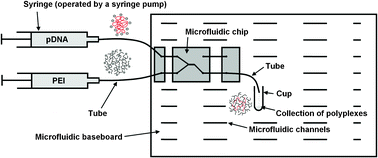 Researchers at Philipps-Universität Marburg, Germany, have developed a new technique for the production of polyplexes that produces more regular sized complexes compared to the standard pipetting methods. Polyplexes are the complexes that form by electrostatic interactions of oppositely charged macromolecules – in this case poly(ethylene imine) (PEI) and plasmid DNA, which are used in gene therapy applications.
Researchers at Philipps-Universität Marburg, Germany, have developed a new technique for the production of polyplexes that produces more regular sized complexes compared to the standard pipetting methods. Polyplexes are the complexes that form by electrostatic interactions of oppositely charged macromolecules – in this case poly(ethylene imine) (PEI) and plasmid DNA, which are used in gene therapy applications.