Microfluidics can be used to trap a single DNA-enzyme complex in its native state for real-time analysis without having to immobilise the DNA or the enzyme, claim US researchers.
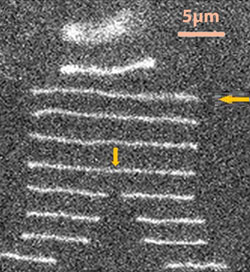
Enzymes called restriction enzymes are used to chop up DNA at specific points called recogition sites, making them useful tools in biochemistry. To anayse how they recognise and cleave DNA, the enzyme or DNA needs to be immobilised on a glass slide, but this can modify their properties, and make it difficult to analyse the products. To combat this, Susan Muller and Weilin Xu at the University of California, Berkeley, pre-bound a restriction enzyme to DNA, and fed it through a microfluidic system. This trapped the complex, and then stretched it out. Adding Mg2+ then activated the enzyme, cleaving the DNA, and permitting analysis of the products.
Ron Larson, a chemical engineering expert at the University of Michigan, Ann Arbor, US, says: ‘this work represents a novel and elegant use of fluidics to trap and stretch single DNA molecules without interference by surfaces.’ He adds that ‘the “look Ma, no hands” approach pursued by Xu and Muller has a number of advantages, not least of which is the ability to recover cleavage products for further study.’
Link to journal article
Exploring both sequence detection and restriction endonuclease cleavage kinetics by recognition site via single-molecule microfluidic trapping
Weilin Xu and Susan J. Muller, Lab Chip, 2011
DOI: 10.1039/c0lc00176g