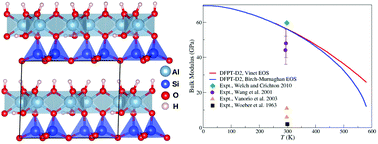
Professor Robert (Bob) Williams died this March at the age of 89. He was a true pioneer in the field of bio-inorganic chemistry – especially concerning the role of calcium as a biological messenger – and contributed substantially to our understanding of the evolution of life. Professor Williams was often considered as one of the first people to start thinking about metallomics as a field, and will be greatly missed amongst his peers.
Professor Robert (Bob) Williams died this March at the age of 89. He was a true pioneer in the field of bio-inorganic chemistry – especially concerning the role of calcium as a biological messenger – and contributed substantially to our understanding of the evolution of life. Professor Williams was often considered as one of the first people to start thinking about metallomics as a field, and will be greatly missed amongst his peers.
In memory of Professor Williams’ huge contribution to the field, we have collated a number of his publications across Metallomics, Dalton Transactions and ChemComm below. We hope you enjoy revisiting some of his exceptional work.
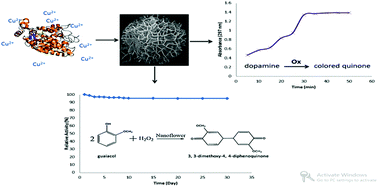
Copper proteomes, phylogenetics and evolution, L. Decaria, I. Bertini, R.J.P. Williams, Metallomics, 2011, 56–60
Zinc proteomes, phylogenetics and evolution, L. Decaria, I. Bertini, R.J.P. Williams, Metallomics, 2010, 706–709
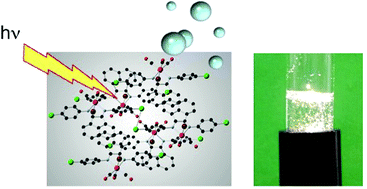
A chemical systems approach to evolution, R.J.P. Williams, Dalton Transactions, 2007, 991–1001
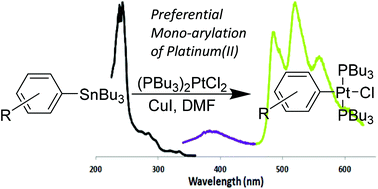
Metallo-enzyme catalysis, R.J.P. Williams, Chemical Communications, 2003, 1109–1113
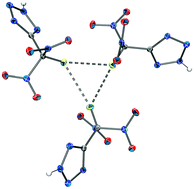
The chemical elements of life, R.J.P. Williams, Journal of the Chemical Society, Dalton Transactions, 1991, 539–546
Temperature study of the solution conformations of aqueous lanthanide(III) complexes containing monodentate ligands, A.L. Du Preez, S. Naidoo, R.J.P. Williams, Journal of the Chemical Society, Dalton Transactions, 1988, 2315–2321
A proton NMR study of some CoII complexes containing the N-hexadecyl-iminodiacetate ligand, C.J. Rix, R.J.P. Williams, Journal of the Chemical Society, Chemical Communications, 1986, 203–205
Solution conformation of aqueous lanthanide(III)-antipyrine complexes, A.L. Du Preez, R.J.P. Williams, Journal of the Chemical Society, Dalton Transactions, 1986, 1425–1429
Precipitation within unilamellar vesicles. Part 1. Studies of silver(I) oxide formation, S. Mann, R.J.P. Williams, Journal of the Chemical Society, Dalton Transactions, 1983, 311–316
Precipitation within unilamellar vesicles. Part 2. Membrane control of ion transport, S. Mann, M.J. Kime, R.G. Ratcliffe, R.J.P. Williams, Journal of the Chemical Society, Dalton Transactions, 1983, 771–774
The characterisation of the nature of silica in biological systems, S. Mann, C.C. Perry, R.J.P. Williams, C.A. Fyfe, G.C. Gobbi, G.J. Kennedy, Journal of the Chemical Society, Chemical Communications, 1983, 168–170
New organo-metallic reagents for electron microscopy, S. Mann, R.J.P. Williams, P.R. Sethuraman, M.T. Pope, Journal of the Chemical Society, Chemical Communications, 1981, 1083–1084
Solid state phosphorus NMR spectroscopy of minerals and soils, R.J.P. Williams, R.G.F. Giles, A.M. Posner, Journal of the Chemical Society, Chemical Communications, 1981, 1051–1052
Electron relaxation rates of lanthanide aquo-cations, B.M. Alsaadi, F.J.C. Rossotti, R.J.P. Williams, Journal of the Chemical Society, Dalton Transactions, 1980, 2147–2150
Hydration of complexone complexes of lanthanide cations, B.M. Alsaadi, F.J.C. Rossotti, R.J.P. Williams, Journal of the Chemical Society, Dalton Transactions, 1980, 2151–2154
Preparation of Ag2O crystallites within phospholipid vesicles and their use in nucleation studies, J.L. Hutchison, S. Mann, A.J. Skarnulis, R.J.P. Williams, Journal of the Chemical Society, Chemical Communications, 1980, 634–635
Studies of lanthanide (III) dipicolinate complexes in aqueous solution. Part 2. Hydration, B.M. Alsaadi, F.J.C. Rossotti, R.J.P. Williams, Journal of the Chemical Society, Dalton Transactions, 1980, 813–816
Studies of lanthanide(III) pyridine-2,6-dicarboxylate complexes in aqueous solution. Part 1. Structures and 1H nuclear magnetic resonance spectra, B.M. Alsaadi, F.J.C. Rossotti, R.J.P. Williams, Journal of the Chemical Society, Dalton Transactions, 1980, 597–602
Location of biological compartments by high resolution NMR spectroscopy and electron microscopy using magnetite-containing vesicles, S. Mann, A.J. Skarnulis, R.J.P. Williams, Journal of the Chemical Society, Chemical Communications, 1979, 1067–1068
Mapping organic molecules in biological space by high resolution NMR spectroscopy and electron microscopy, A.J. Skarnulis, P.J. Strong, R.J.P. Williams, Journal of the Chemical Society, Chemical Communications, 1978, 1030–1032
An investigation of some potential uses of the gadolinium(III) ion as a structural probe, E.C.N.F. Geraldes, R.J.P. Williams, Journal of the Chemical Society, Dalton Transactions, 1977, 1721–1726
Structure of lanthanide(III) mono- and bis-dipicolinates in solution, B.M. Alsaadi, F.J.C. Rossotti, R.J.P. Williams, Journal of the Chemical Society, Chemical Communications, 1977, 527–529
Assignment of the NMR spectrum of iron(III) protoporphyrin IX dicyanide using paramagnetic shift and broadening probes, J.G. Brassington, R.J.P. Williams, P.E. Wright, Journal of the Chemical Society, Chemical Communications, 1975, 338–340
Conformational studies of peroxidase-substrate complexes. Structure of the indolepropionic acid-horseradish peroxidase complex, P.S. Burns, R.J.P. Williams, P.E. Wright, Journal of the Chemical Society, Chemical Communications, 1975, 795–796
The temperature dependence of some physical properties of cobinamides and cobalamins, S.A. Cockle, O.D. Hensens, H.A.O. Hill, R.J.P. Williams, Journal of the Chemical Society, Dalton Transactions, 1975, 2633–2634
Conformational studies of lanthanide complexes with carboxylate ligands, B.A. Levine, J.M. Thornton, R.J.P. Williams, Journal of the Chemical Society, Chemical Communications, 1974, 669–670
Ethylenediaminetetra-acetato-lanthanate(III), -praesodimate(III), -europate(III), and -gadolinate(III) complexes as nuclear magnetic resonance probes of the molecular conformations of adenosine 5′- monophosphate and cytidine 5′-monophosphate in solution, C.M. Dobson, R.J.P. Williams, A.V. Xavier, Journal of the Chemical Society, Dalton Transactions, 1974, 1762–1764
Intramolecular nuclear Overhauser effects in proton magnetic resonance spectra of proteins, I.D. Campbell, C.M. Dobson, R.J.P. Williams, Journal of the Chemical Society, Chemical Communications, 1974, 888–889
Lanthanoid(III) cations as nuclear magnetic resonance conformational probes: Studies on cytidine 5′-monophosphate at pH 2, C.D. Barry, C.M. Dobson, R.J.P. Williams, A.V. Xavier, Journal of the Chemical Society, Dalton Transactions, 1974, 1765-1769
Nuclear magnetic resonance spectra of dimeric cupric compounds, W. Byers, R.J.P. Williams, Journal of the Chemical Society, Dalton Transactions, 1973, 555–560
Separation of contact and pseudo-contact contributions to shifts induced by lanthanide(III) ions in nuclear magnetic resonance spectra, C.M. Dobson, R.J.P. Williams, A.V. Xavier, Journal of the Chemical Society, Dalton Transactions, 1973, 2662–2664
The effect of 1,3,5-trinitrobenzene on 1H nuclear magnetic resonance and electron paramagnetic resonance spectra of some cobalt(II) porphyrins, H.A.O. Hill, P.J. Sadler, R.J.P. Williams, Journal of the Chemical Society, Dalton Transactions, 1973, 1663–1667
Origin of lanthanide nuclear magnetic resonance shifts and their uses, B. Bleaney, C.M. Dobson, B.A. Levine, R.B. Martin, R.J.P. Williams, A.V. Xavier, Journal of the Chemical Society, Chemical Communications, 1972, 791b–793
The chemistry of vitamin B12. Part XVI. Binding of thiols to the cobalt(II) corrins, S. Cockle, H.A.O. Hill, S. Ridsdale, R.J.P. Williams, Journal of the Chemical Society, Dalton Transactions, 1972, 297–302
A method of assigning 13C nuclear magnetic resonance spectra using europium(III) ion-induced pseudocontact shifts and C-H heteronuclear spin decoupling techniques, B. Birdsall, J. Feeney, J.A. Glasel, R.J.P. Williams, A.V. Xavier, Journal of the Chemical Society D: Chemical Communications, 1971, 1473–1474
Methylation by methyl vitamin B12, G. Agnes, S. Bendle, H.A.O. Hill, F.R. Williams, R.J.P. Williams, Journal of the Chemical Society D: Chemical Communications, 1971, 850–851
Kinetics of substitution of co-ordinated carbanions in cobalt(III) corrinoids, H.A.O. Hill, J.M. Pratt, S. Ridsdale, F.R. Williams, R.J.P. Williams, Journal of the Chemical Society D: Chemical Communications, 1970, 341
Thallium(I) as a potassium probe in biological systems, J.P. Manners, K.G. Morallee, R.J.P. Williams, Journal of the Chemical Society D: Chemical Communications, 1970, 965–966
The lanthanide cations as nuclear magnetic resonance probes of biological systems, K.G. Morallee, E. Nieboer, F.J.C. Rossotti, R.J.P. Williams, A.V. Xavier, R.A. Dwek, Journal of the Chemical Society D: Chemical Communications, 1970, 1132–1133