Hypochlorous acid (HOCl) is a weak acid, formed from the reaction of chlorine with water. In addition to its use as a reagent in organic chemistry, it has significant biological relevance. HOCl is generated in biological systems in a reaction between chloride ions and hydrogen peroxide, catalysed by the enzyme myeloperoxidase.
This enzyme is secreted by phagocytes (cells which help protect the body by ‘ingesting’ bacteria) when they are activated during an immune response. Hypochlorite (ClO–), the conjugate base of HOCl, is extremely toxic to bacteria and plays a vital role in assisting the activated phagocytes with killing a wide range of pathogens.1
Excess production of HOCl in a living system can have a detrimental effect, as HOCl can react with many different biological molecules, including DNA, cholesterol and proteins, leading to changes in their biological properties. An example of this is the reaction of hypochlorous acid with unsaturated bonds in lipids, which produces a species called a chlorohydrin. This disrupts the formation of the essential lipid by-layers which form around cells.
Excess hypochlorous acid has been implicated in conditions such as inflammatory diseases, neurodegeneration and cancers.2 In order to fully understand the role of HOCl in these biological processes, accurate detection methods must be developed to monitor the molecule in living cells.
Several ‘HOCl-recognising’ molecules have been found to be effective sensors of hypochlorous acid. When conjugated with a fluorophore, these probes can successfully ‘recognise’ HOCl by reacting with it, however their application in vivo is still limited due to their excitation wavelengths being in the ultraviolet region of light.3 Sensors with adsorption (or emission) in the visible light range are more desirable for clinical diagnostic applications.
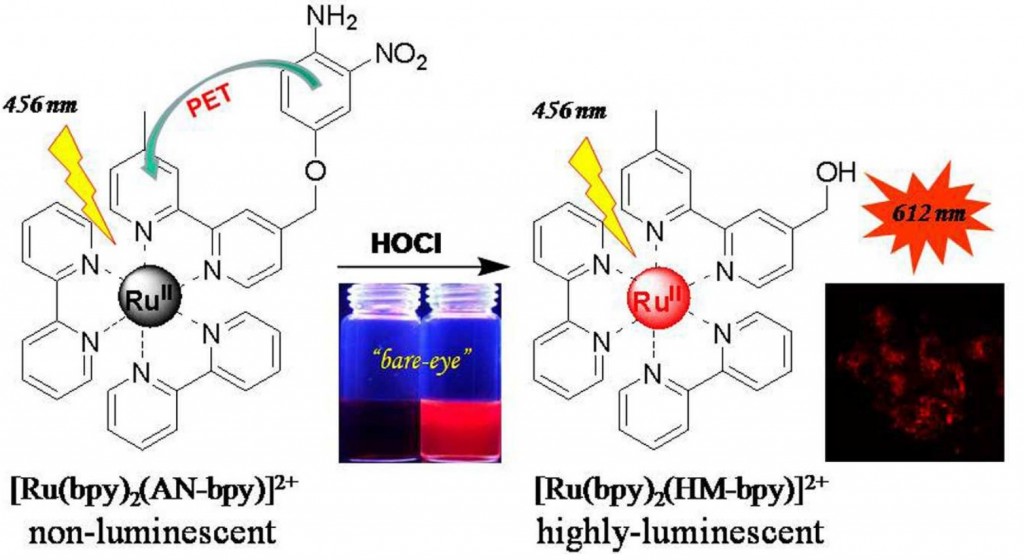
In one recent paper in Dalton Transactions, Yuan and co-workers combine an excellent HOCl-recognising moiety: 4-amino-3-nitrol phenol) and a ruthenium(II)-2,2-bipyridyl complex, which is well known to exhibit visible light adsorption and emission, into one compound to create a luminescent probe for HOCl.
The resulting complex [Ru(bpy)2(AN-bpy)][PF6]2 is very weakly luminescent but, upon reaction with HOCl in aqueous media, converts to [Ru(bpy)2(HM-bpy)][PF6]2, which has a luminescence signal which is 110-fold stronger.
Impressively, the authors show that when HeLa cells are incubated with [Ru(bpy)2(AN-bpy)][PF6]2 for two hours they remain non-luminescent; when the same cells are subsequently treated with HOCl for thirty minutes, a bright red luminescence is observed, clearly demonstrating the potential for using this ruthenium complex as an in vivo, luminescent detector of hypochlorous acid.
To find out more, read the article using the link below:
Development of a functional ruthenium(II) complex for probing hypochlorous acid in living cells
Dalton Trans. 2014, DOI:10.1039/C4DT00179F
 |
Dr C. Liana Allen is currently a post-doctoral research associate in the group of Professor Scott Miller at Yale University, where she works on controlling the enantio- or regioselectivity of reactions using small peptide catalysts. Liana received her Ph.D. in organic chemistry at Bath University with Professor Jonathan Williams, where she worked on developing novel, efficient syntheses of amide bonds.
|
References
1 J. M. Albrich, C. A. McCarthy, J. K. Hurst, Prot. Nat. Acad. Sci., 1981, 78, 210.
2 T. I. Kim, S. Park, Y. Choi, Y. Kim, Chem.-Asian J., 2011, 6, 1358
3 Y. Xiao, R. Zhang, Z. Ye, Z. Dai, H. An, J. Yuan, Anal. Chem., 2012, 84, 10785.
Comments Off on On the Hunt for HOCl