Declan Gaynor and Darren Griffith discuss how medicinal inorganic chemistry is currently flourishing in this Dalton Transactions Perspective. Understanding the role of metals in biological systems is very important for drug design; the predictability and control of inorganic complexes make fine-tuning the properties of drugs incorporating such complexes a real possibility. Metal-based compounds are already routinely administered on a regular basis and this Perspective encourages chemists to further investigate inorganic therapeutic and diagnostic medicine by looking at previous successes, e.g. MRI contrast agents, then moving onto current challenges such as antibacterial compounds for tackling hospital acquired infections.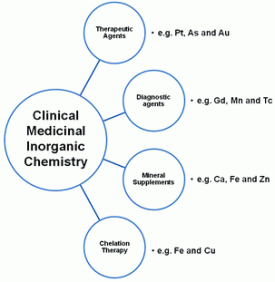
To find out more, you can download the Perspective now – which is free to access for 4 weeks!
The prevalence of metal-based drugs as therapeutic or diagnostic agents: beyond platinum
Declan Gaynor and Darren M. Griffith
Dalton Trans., 2012
DOI: 10.1039/C2DT31601C, Perspective
Also of interest…
Mn(II) complexes of novel hexadentate AAZTA-like chelators: a solution thermodynamics and relaxometric study
Lorenzo Tei, Giuseppe Gugliotta, Marianna Fekete, Ferenc K. Kálmán and Mauro Botta
Dalton Trans., 2011, 40, 2025-2032
DOI: 10.1039/C0DT01114B, Paper
Metallic radionuclides in the development of diagnostic and therapeutic radiopharmaceuticals
Sibaprasad Bhattacharyya and Manish Dixit
Dalton Trans., 2011, 40, 6112-6128
DOI: 10.1039/C1DT10379B, Perspective
From themed issue Radiopharmaceuticals for imaging and therapy
The status of platinum anticancer drugs in the clinic and in clinical trials
Nial J. Wheate, Shonagh Walker, Gemma E. Craig and Rabbab Oun
Dalton Trans., 2010, 39, 8113-8127
DOI: 10.1039/C0DT00292E, Perspective
Are you following us on Twitter? @DaltonTrans












 A
A