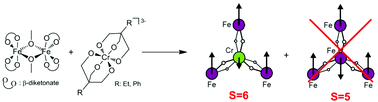
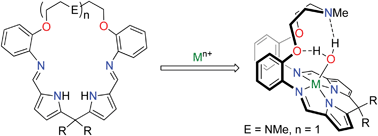
Scientists in Italy and Brazil report a new synthesis for making heteronuclear propeller-like single molecule magnets (SMMs) that could be used to design structures with a variety of d- and f-block metals and R groups, yielding 100% pure complexes without metal scrambling.
There is considerable interest in manipulating SMMs to have higher ground spin states, enhanced magnetic properties and increased robustness for possible future applications in quantum computing. In this study, Roberta Sessoli and colleagues use a central chromium(III) ion in their synthesis of Fe3Cr propeller-like aggregates to show that molecular ground spin state can be altered by selectively changing the spin state of the metal ion.
The chemically inert chromium(III) produces a kinetically stable central “core” which then combines with the dimeric iron(III) starting material to give propeller-like complexes
The synthetic method could potentially be tailored to produce propeller-like SMMs with particular properties.
Read more about the synthesis for free at…
A new approach to the synthesis of heteronuclear propeller-like single molecule magnets
Pasquale Totaro, Kátia Cristina M. Westrup, Marie-Emmanuelle Boulon, Giovana G. Nunes, Davi F. Back, Andersson Barison, Samuele Ciattini, Matteo Mannini, Lorenzo Sorace, Jaísa F. Soares, Andrea Cornia and Roberta Sessoli
Dalton Trans., 2013
DOI: 10.1039/C2DT32618C
Here are some other Dalton Transactions articles by the same authors:
Lanthanides in molecular magnetism: so fascinating, so challenging
Javier Luzon and Roberta Sessoli
Dalton Trans., 2012, 41, 13556-13567
DOI: 10.1039/C2DT31388J, Perspective
From themed issue Frontier and Perspectives in Molecule-Based Quantum Magnet
Magnetic and optical bistability in tetrairon(III) single molecule magnets functionalized with azobenzene groups
Thazhe Kootteri Prasad, Giordano Poneti, Lorenzo Sorace, Maria Jesus Rodriguez-Douton, Anne-Laure Barra, Petr Neugebauer, Luca Costantino, Roberta Sessoli and Andrea Cornia
Dalton Trans., 2012, 41, 8368-8378
DOI: 10.1039/C2DT30172E, Paper
Dimers and chains of {3d–4f} single molecule magnets constructed from heterobimetallic tectons
Traian D. Pasatoiu, Mael Etienne, Augustin M. Madalan, Marius Andruh and Roberta Sessoli
Dalton Trans., 2010, 39, 4802-4808
DOI: 10.1039/B925425K, Paper
From themed issue Molecular magnets
Are you following us on Twitter? @DaltonTrans