Prof. Evamarie Hey-Hawkins and Dalton Transactions
This year, we are celebrating the 50th volume of Dalton Transactions by taking a look at some of our authors who have published over 50 articles in the journal. This week we learn what Dalton Transactions means to Professor Evamarie Hey-Hawkins.
Our author at a glance:
Prof. Dr. Evamarie Hey-Hawkins is based at the Institute of Inorganic Chemistry in the Faculty of Chemistry and Mineralogy at Leipzig University, Germany. Her research is focused on three main areas: homogeneous transition metal catalysis; inorganic compounds for medicinal and biological applications; and phosphorus-rich novel materials. She chooses to publish in Dalton Transactions because of its “excellent reputation and high-quality, original publications”, and has always felt that her research and contributions are highly appreciated.
Please can you summarise your most recent research published in Dalton Transactions?
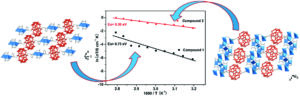
In stimuli-responsive catalysis, redox-switchable catalysis is an important area. In our latest publication, we have shown that the aromatic core (s-triazine, benzene, or trifluorobenzene) in C3-symmetric tris(ferrocenyl)arene-based tris-phosphanes has a pronounced effect on their coordination behaviour towards gold(I), resulting in two different coordination modes for the 1:1 and 2:3 (L:M) complexes, respectively. The redox-responsive nature of the complexes was exploited in the catalytic ring-closing isomerisation of N-(2-propyn-1-yl)benzamide, in which the benzene-based 2:3 (L:M) complex comprising six ferrocenylene moieties was shown to display multiple activity states depending on the degree of (reversible) oxidation.
How do you intend to expand upon your research in the future?
As my scientific interests are manifold, expansion of my research in the future will be along our three main research areas. In switchable catalysis, we will focus on photo-switchable catalytic systems based on phosphines. In medicinal chemistry, we are now targeting anti-cancer drugs comprising more than one active component, to overcome resistance and reduce side effects. And last but not least, in materials science, we are now looking at the targeted synthesis of higher oligophosphines (≥ 9 phosphorus atoms), such as nona-, deca- or even higher oligophosphines, and their metal complexes as precursors for novel phosphorus-rich metal phosphides.
What would you say are the biggest barriers which need to be overcome to expand your research?
Our research is highly interdisciplinary, and thus requires collaboration with experts worldwide. For example, in photo-switchable catalysis, we need to collaborate with experts in photochemistry and photophysics to understand the switching mechanisms and how to influence them. In medicinal chemistry, we are preparing the potential drugs, but we need experts to test their anti-tumour activity and to help us understand their mode of action. Luckily, we are extremely well connected, but the present pandemic has made personal interactions quite difficult for the time being.
You’ve published over 50 articles in Dalton Transactions; which of these works do you find to be most interesting/significant for our broad inorganic audience?
Our 2007 paper on Aminoalkylferrocenyldichlorophosphanes: facile synthesis of versatile chiral starting materials. As the title states, this ferrocenyl dichlorophosphine can be employed as an extremely versatile starting material for a large library of P-chiral, planar-chiral ferrocene derivatives.
Our joint paper on Novel chiral 1,5-diaza-3,7-diphosphacyclooctane ligands and their transition metal complexes with Andrey A. Karasik and his team from the Arbusov Institute in Kazan, Russia, in 2003, was the first of a series of papers on P,N heterocycles, macrocycles and cryptands. These ligands show a very rich coordination chemistry, and the resulting complexes are not only interesting for catalytic applications, but also as luminescent materials.
Our 2004 publication on The reactivity of cyclo-(P5tBu4)− towards group 13, 14 and 15 metal chlorides: complexation and formation of cyclooligophosphanes, {cyclo-(P5tBu4)}2 and {cyclo-(P4tBu3)PtBu}2, by reductive elimination opened the door for the targeted formation of phosphorus-rich oligophosphines.
Outside of your own research, please suggest a Dalton Transactions article which you think has made a significant contribution to its field?
It would be rather difficult to name just one article. However, I think that the Themed Collections, especially the annual “Frontier and Perspective articles” are an excellent collection of the most significant contributions in their respective fields.
What advice do you have for young researchers new to your field?
Be enthusiastic about your research. Believe in your ideas, have courage, determination, and perseverance in following your scientific goals. Collaboration can make your scientific life much richer but select your collaborators carefully. Be committed to very good science and of course, good scientific practice. Think positive and enjoy life.
What does Dalton Transactions mean to you?
I published my first paper in Dalton Transactions thirty years ago in 1991 on the use of zirconocene(IV) bis(phosphanido) complexes as PR2 transfer reagents, an interdisciplinary topic that I worked on during my habilitation and is still topical today. While it took another ten years before I submitted my second paper dealing with the syntheses and solid-state structures of primary alkali metal phosphanides (MPHR, with M = K and Rb) in 2001, many more publications followed. I have always felt that my research and my contributions were highly appreciated by the journal.
Why do you choose to publish in Dalton Transactions?
Dalton Transactions has an excellent reputation and is known for high-quality, original publications. As the scope of Dalton Transactions is perfectly aligned with my various research interests in inorganic, organometallic and bioinorganic chemistry, including new inorganic materials and homogeneous catalysis, I can be sure to reach the readership that we want to target with our publications in various areas.
What is your experience of publishing with Dalton Transactions?
The handling of my submitted manuscripts is always very professional, fast and highly competent. After acceptance, publication is also very fast.
You can check out Eva’s most recent Dalton Transactions article on how arene substitution affects the coordination and catalytic behaviour of tris(1-phosphanyl-1′-ferrocenylene)arene gold(I) complexes below.
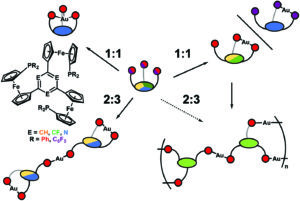 The core of the matter – arene substitution determines the coordination and catalytic behaviour of tris(1-phosphanyl-1′-ferrocenylene)arene gold(I) complexes
The core of the matter – arene substitution determines the coordination and catalytic behaviour of tris(1-phosphanyl-1′-ferrocenylene)arene gold(I) complexes
Axel Straube, Peter Coburger, Marvin Michak, Mark R. Ringenberg and Evamarie Hey-Hawkins*
Dalton Trans., 2020, 49, 16667-16682
Check out the full collection of recent research published in Dalton Transactions by all of our featured Golden Authors in our Celebrating our Golden Authors collection.















 eactions of the C–B–N-substituted borinium cation [MesBNiPr2]+
eactions of the C–B–N-substituted borinium cation [MesBNiPr2]+