Gold nanoparticles (Au-NPs) are attractive for applications in important technological fields as components of biosensors, novel catalysts and new optical, magnetic or electrical devices. Traditional techniques for their preparation include thermal decomposition of organometallic precursors, chemical reduction of Au(I) ions, and electrochemical and photochemical processes. Methods based on reduction by sodium borohydride or citrate ions have been used successfully as the reducing agent acts also as a stabilizer and prevents further aggregation.
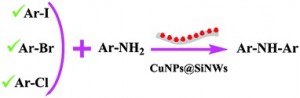
 As an alternative, Heinrich Lang, from Chemnitz University of Technology, and coworkers have developed a family of gold(I) carboxylate complexes which can be used as precursors for the preparation of Au-NPs in a single step. The ligand works as the reducing and stabilizing part, simultaneously, in one molecule. After thermally induced decomposition of the complexes, Au-NPs are obtained with sizes in the range from 3.3 to 6.5 nm, and almost homogenous size distribution. The ethylene glycol-functionalized carboxylates were obtained from easy chemical reactions using available ethylene gycol or directly from commercial suppliers, and the complexes obtained by a two step procedure. The authors report than larger chain lengths increased stability and slowed particle growth.
As an alternative, Heinrich Lang, from Chemnitz University of Technology, and coworkers have developed a family of gold(I) carboxylate complexes which can be used as precursors for the preparation of Au-NPs in a single step. The ligand works as the reducing and stabilizing part, simultaneously, in one molecule. After thermally induced decomposition of the complexes, Au-NPs are obtained with sizes in the range from 3.3 to 6.5 nm, and almost homogenous size distribution. The ethylene glycol-functionalized carboxylates were obtained from easy chemical reactions using available ethylene gycol or directly from commercial suppliers, and the complexes obtained by a two step procedure. The authors report than larger chain lengths increased stability and slowed particle growth.
To find out more about it, read the full paper in Dalton Transactions.
Gold nanoparticles generated by thermolysis of “all-in-one” gold(I) carboxylate complexes
André Tuchscherer, Dieter Schaarshmidt, Steffen Schulze, Michael Hietschold, Heinrich Lang,
Dalton Transactions, 2012, Advance Article
DOI: 10.1039/c2dt11748g