We are delighted to announce our new spotlight collection on Fluorinated ligands. Spotlight Collections are ongoing themed collections highlighting the best past and present work in Dalton Transactions.
This collection focuses on fluorinated ligands and their effects on physical properties and chemical reactivity.
Fluorine embodies reactivity and inertness, both of which are exploited by inorganic chemists. It is the most electronegative element in the periodic table (3.98 on the Pauling scale) and has a remarkably high reduction potential (2.87 V). The van der Waals and covalent radii of fluorine are quite short (ranked third, only after the first-row elements hydrogen and helium), making it unusually small for its atomic number. The homonuclear bond in F2 is particularly reactive while some heteronuclear bonds involving fluorine are remarkably stable. The combined effects of these features on fluorine-containing molecules are often unmatched by any other element, for example with carbon, where it forms very robust, chemically inert single bonds; the BDE of C-F compared to C-H bonds in CF4 and CH4 are 546.8 and 439.3 kJ/mol, respectively.
Metal complexes of fluorinated ligands in comparison to their non-fluorinated, hydrocarbon counterparts, usually display different properties such as relatively high thermal and oxidative stability, volatility, diminished vibrational modes and shifted spectroscopic features (absorption and emission), as well as unique reactivity profiles. They are also ideal for applications in fluorous-biphase media and supercritical CO2. Additionally, fluorine containing anions such as [BF4]–, [SbF6]–, [B(C6F5)4]−, and [B{3,5-(CF3)2C6H3}4]− are among the weakest donors known. This Spotlight collection brings together contributions by leading researchers on this topic to highlight some of the fascinating developments in fluorinated supporting ligands, C-F inertness, electron-withdrawing effects of fluoro-substituents, the weakly-coordinating nature of fluorinated anions, efforts to functionalize C-F bonds, and species that selectively deliver F, F· and F–.
This collection is guest edited by Dalton Transactions Advisory Board member Professor Rasika Dias, The University of Texas at Arlington, USA, and Professor Linda Doerrer, Boston University, USA.
Professor Linda Doerrer |
Professor Rasika Dias |
See the full collection as it grows on our collection webpage, and check out a selection of articles below:
|
Florian Morsbach, Steffen Klenner, Rainer Pöttgen and Walter Frank*
|
 |
 |
Max Klotzsche, Davide Barreca*, Lorenzo Bigiani, Roberta Seraglia, Alberto Gasparotto, Laura Vanin, Christian Jandl, Alexander Pöthig, Marco Roverso, Sara Bogialli, Gloria Tabacchi*, Ettore Fois, Emanuela Callone, Sandra Dirè and Chiara Maccato Dalton Trans., 2021, 50, 10374-10385 |
|
Çetin Çelik, Nazli Farajzadeh, MustafaAkın, Göknur Yaşa Atmaca, Özgül Sağlam, Neslihan Şaki*, Ali Erdoğmuş* and Makbule Burkut Koçak* Dalton Trans., 2021, 50, 2736-2745 |
 |
 |
Iridium complexes of an ortho-trifluoromethylphenyl substituted PONOP pincer ligand (Open Access)
Ethan W. Poole, Itxaso Bustos, Thomas M. Hood, Jennifer E. Smart and Adrian B. Chaplin* Dalton Trans., 2023, 52, 1096-1104 |
|
From ferrocene to 1,2,3,4,5-pentafluoroferrocene: halogen effect on the properties of metallocene William Erb*, Nicolas Richy, Jean-Pierre Hurvois*, Paul J. Low* and Florence Mongin Dalton Trans., 2021, 50, 16933-16938 |
 |
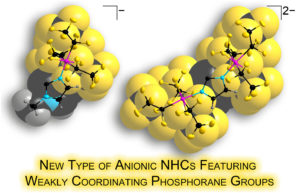
| Anionic N-heterocyclic carbenes featuring weakly coordinating perfluoroalkylphosphorane moieties |