Geographer Vaclav Smil described the Haber-Bosch process as the “detonator of the human population explosion” in the twentieth century, in his Nature Millennium Essay.1 Today, nearly 80% of nitrogen atoms in human tissue have been through the Haber-Bosch process;2 where nitrogen gas is converted to ammonia converted into industrial fertilizers.
The Haber-Bosch process has now entered its second century. High temperatures and pressures and a catalyst composed of magnetite (Fe3O4), wüstite (FeO) and iron(0) metal, push the equilibrium of a mixture of pure hydrogen, nitrogen and ammonia gas towards the formation of ammonia. Today, one of the greatest challenges of industrial chemistry is to find an alternative catalyst and process.
In 1991 Leigh et. al. reported the nitrogen of nitrogen by a homogeneous Fe complex with two chelating phosphine ligands.3 They were able to reduce N2 to ammonia (isolated as NH4+) under strongly acidic conditions. However, following this discovery, verification and mechanistic questions remained.
|
|
|
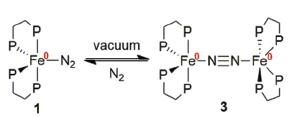
| The previously unreported dimer |
In a recent article, ‘Teaching old compounds new tricks: efficient N2 fixation by simple Fe(N2)(diphosphine)2 complexes‘ published in Dalton Transactions, , Ashley and co-workers report their investigation of the Leigh compound. They have persued a peak that was previously unaccounted for in the 31P NMR spectrum which has led them to isolate a unique dimer of this complex, bridged by molecular N2. Comparing the reactivities of this dimer with the two monomers that feature different simple chelating phosphine ligands, they unambiguously report yields of NH3 and N2H4 after reaction with triflic acid, and discern dependences based on ligand, temperature, and solvent.
This hitherto unreported dimeric compound, and the impressive NH3/N2H4 yields achieved with the monomers tested, add a significant piece to the puzzle of how iron-mediated N2 activation occurs.
Read the full article here:
Teaching old compounds new tricks: efficient N2 fixation by simple Fe(N2)(diphosphine)2 complexes
Laurence R. Doyle, Peter J. Hill, Gregory G. Wildgoose and Andrew E. Ashley
Dalton Trans., 2016, Advance Article
DOI: 10.1039/C6DT00884D
1V. Smil Nature 1999, 400, 415.
2R. W. Howarth Harmful Algae 2008, 8, 14.
3J. G. Leigh and M. Jimenez-Tenorio, J. Am. Chem. Soc., 1991, 113, 5862.