To me the most interesting observation in the recent Dalton Transactions paper from the group of Professor Phil Power was their suggestion that secondary interactions between dicoordinate Fe(II) atoms and carbon atoms on their ligands probably have a significant effect on the magnetic moment of the complexes.
Specifically, they postulate that these interactions help to quench the orbital contribution to the magnetic moment, which is significant for other dicoordinate Fe(II) complexes studied.
But let’s take a step back. Dicoordinate Iron(II) complexes were unknown until the 1980’s, thought to be too unstable to isolate and structurally characterize. As the authors detail, examples were discovered gradually. All featuring large coordinating ligands bound through anionic C, N, or O donors. Power reports a total of thirty currently known.
No one, it appears, has previously undertaken thorough magnetic studies. Indeed, do you remember studying how crystal field theory applies to dicoordinate metal species in your introductory inorganic class? I don’t.
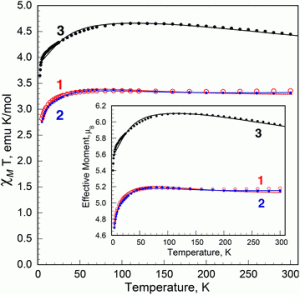
The authors focus their attention on four species. Two of these feature large silylamido ligands and have solid-state N-Fe-N angles of 169o and 172o, the other have two large aryl ligands and exhibit slightly more bent geometries. The authors support the evidence that a significant part of the measured temperature-dependent magnetic moment of these molecules arises from the orbital contribution – that is, from the motion of electrons around the iron nucleus, rather than arising only from the spin contribution, the electrons spinning about their own axes.
However, the less linear aryl iron(ll) complexes show the greater orbital contribution to the magnetic moment, which brings me back to the beginning. This is a thorough paper; the authors also construct a spectrochemical series for the dicoordinate Fe(II) complexes and exactingly compare computed and experimental magnetic data. But the original small structure-function observation fascinated me on my first reading.
Read the full article now:
Ligand field influence on the electronic and magnetic properties of quasi-linear two-coordinate iron(II) complexes
Nicholas F. Chilton, Hao Lei, Aimee M. Bryan, Fernande Grandjean, Gary J. Long and Philip P. Power
Dalton Trans., 2015, 44, 11202-11211
DOI: 10.1039/C5DT01589H
 |
Ian Mallov is currently a Ph.D. student in Professor Doug Stephan’s group at the University of Toronto. His research is focused on synthesizing new Lewis-acidic compounds active in Frustrated Lewis Pair chemistry. He grew up in Truro, Nova Scotia and graduated from Dalhousie University and the University of Ottawa, and worked in chemical analysis in industry for three years before returning to grad school. |