In fall 2008 I visited Boston, where a good friend gave me a tour of Harvard’s chemical laboratories. Passing by a small office, I saw through the window a thin elderly man in glasses hunched over a desk. He was turned away from me but the sign on his office said William N. Lipscomb.
Since Lipscomb’s astonishing groundwork beginning in the 1940’s, boranes have provided fascinating examples of the diversity and possibilities of chemical bonding. Today, boron’s Lewis acidity is widely exploited in catalysis and Frustrated Lewis Pair (FLP) chemistry.
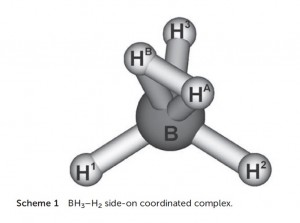
The BH5 molecule, described as BH3 with sigma-bonded dihydrogen bound in an H2 manner, lies at the confluence of many currents of boron chemistry: the activation of hydrogen by FLP’s using borane Lewis acids; three-centre-two-electron bonding; H2 complexes, and the comparison of main group and transition metal chemistry.
In a recent paper in Dalton Transactions, authors Szieberth, Szpisjak, Turczel and Konczol describe BH5 as “rare.” This is an understatement. As they report, its existence was confirmed in 1994 by infra-red spectroscopy in an argon matrix at 10-25K temperatures.
In this paper, they present the modelling of the BH5 complex using Natural Bond Order analysis. Although this has been reported before, the authors use this paper to discern a unique and significant contribution to the stability of η2 H2 borane complexes: the back-donation of electron density from the B-H (or B-R) s-bonds into the σ* orbital of the bound H2, just as electron density from d-orbitals is donated to the H-H σ* orbital in transition metal H2 complexes. Their description of the Lewis structure of BH5 as a BH3/H2 adduct featuring a three-centre-two electron bond accounts for 99.1% of the electron density.
William Lipscomb passed away on April 14, 2011 at the age of 91. But I am sure he would be pleased that work within his research area remains vigorously active.
Read the original paper:
The stability of η2-H2 borane complexes – a theoretical investigation
László Könczöl, Gábor Turczel, Tamás Szpisjaka and Dénes Szieberth
Dalton Trans., 2014,43, 13571-13577
 |
Ian Mallov is currently a Ph.D. student in Professor Doug Stephan’s group at the University of Toronto. His research is focused on synthesizing new Lewis-acidic compounds active in Frustrated Lewis Pair chemistry. He grew up in Truro, Nova Scotia and graduated from Dalhousie University and the University of Ottawa, and worked in chemical analysis in industry for three years before returning to grad school. |