In their recent paper in Dalton Transactions, Erker and co-workers describe B(C6F5)3 as an unorthodox probe for the detection of σ ligand character and allenoid-type bonding in substituted zirconocenes.
Chemists have studied the strong Lewis acid, B(C6F5)3 for the past decade, particularly for its uses in frustrated Lewis pairs (FLPs). This small molecule has, however, gained popularity in other areas of chemistry. In catalysis, B(C6F5)3 is commonly employed to generate cationic metal centres by alkyl group abstraction (σ-ligand abstraction) to activate molecular pre-catalysts for use in polymerisation.
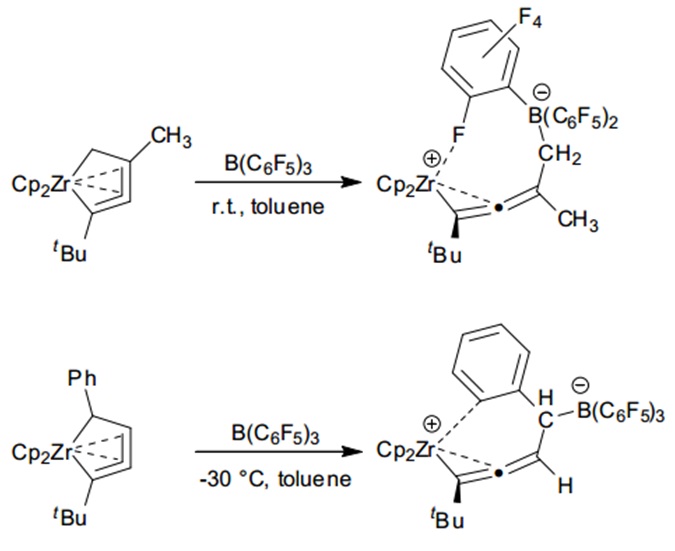
Expanding the scope beyond alkyl groups, Erker and co-workers showed that B(C6F5)3 can mediate cleavage of Zr-C(sp3) bonds in zirconacycles, creating unique allene coordination complexes. In one instance, they used an unsubstituted zirconacycloallenoid (Zr-CH2-) to synthesise a zwitterionic (η2-allenyl)zirconocene with an allene bond angle close to linearity.
In a second case, they reacted a 4-phenyl substituted zirconacycloallenoid (Zr-CHPh-) to produce a zwitterionic allene-coordinated zirconocene.
In demonstrating such reactivity, the authors lead the way for B(C6F5)3 to act as a standard probe for detecting latent σ ligand character in other molecules.
Interested in finding out more? Read the full article:
Reaction of Five-membered Zirconacycloallenoids with the Strong Lewis Acid B(C6F5)3
Gerald Kehr, Gerhard Erker, Constantin Gabriel Daniliuc, Birgit Wibbeling and Georg Bender
Dalton Trans. 2014, DOI: 10.1039/C4DT01137F











