Enzymes function as biological catalysts in a wide array of reactions which are essential for sustaining life. They are almost always large protein molecules which adopt complex three dimensional structures, yet are able to remain structurally dynamic in order to allow the function of the enzyme to be switched on and off.
This feature allows an enzyme’s catalytic activity to be active only when required in the metabolic pathway in which it is involved. For this reason, enzymes have long been a source of inspiration to researchers involved in the design and synthesis of artificial molecular switches and machines.
SARS-CoV 3CLpro is a protease enzyme which is involved in the replication and transcription process of the human coronavirus, the virus which causes severe acute respiratory syndrome (SARS). This enzyme is catalytically inactive as a monomer and only functions as a catalyst when it is bound with another monomer in a complex called a homodimer.
Both crystallographic and mutation studies have implicated several amino acid residues in the dimerization process, however the exact mechanism of dimer formation and how this activates the enzyme’s catalytic activity is still unconfirmed.
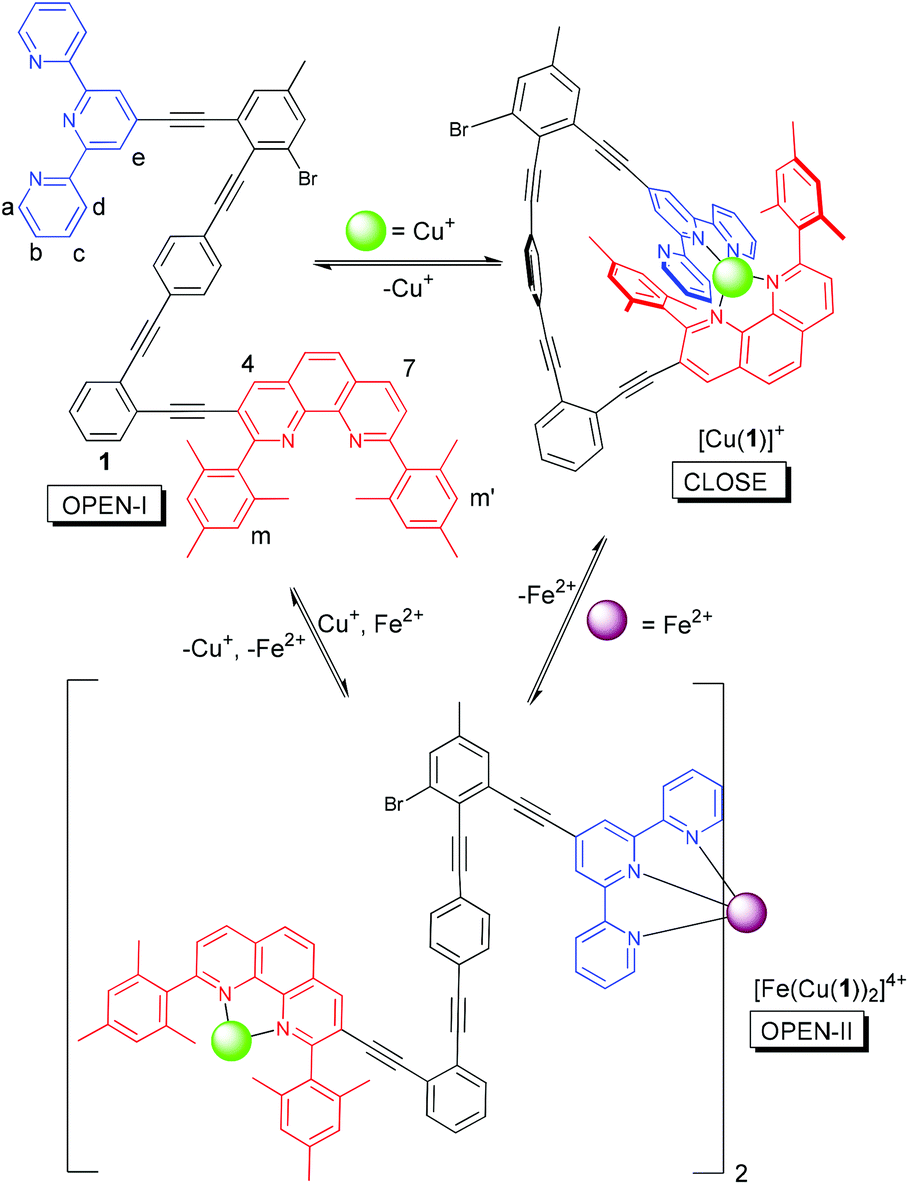
Scheme 1. Switching between open and close conformations of nanoswitch 1 in response to metal ion addition
Drawing inspiration from this monomer-dimer on-off mechanism, Schmittel and co-workers have devised a molecular switch capable of toggling between monomeric and dimeric forms in response to the addition of different metal ions. With no metal ions present, the triangular framework adopts the ‘OPEN-I’ conformation (Scheme 1). Upon the addition of copper(I) ions, the two pyridine-based ligands come into proximity to mutually coordinate the metal.
This changes the conformation of the framework to the ‘CLOSE’ state. Addition of iron(II) ions to the ‘CLOSE’ state again changes the molecular conformation, as the iron(II) ions occupy the terpyridine moiety and the copper(I) ions move to occupy only the shielded phenanthroline ligand.
This both forms a homoleptic dimer, as the iron(II) ions can coordinate two terpyridine moieties simultaneously from two different molecules, and creates a coordinatively unsaturated copper species which acts as a catalyst for a cyclopropanation reaction. The authors impressively demonstrate the reversibility of each step, with the removal of metal ions changing the framework back to its uncoordinated conformation.
In this way, they have successfully made a molecular switch which responds to a metal ion signal (Fe2+) and turns a catalytic complex on and off. Like SARS-CoV 3CLpro enzyme, the catalytically active state is the homodimer, with the monomeric form being catalytically inactive.
To find out more, read the full article:
A monomer-dimer nanoswitch that mimics the working principle of the SARS-CoV 3CLpro enzyme controls copper-catalysed cyclopropanation
Soumen De, Susnata Pramanik and Michael Schmittle
Dalton Trans. 2014, DOI:10.1039/c4dt01508h











