Posted on behalf of Ian Mallov, web writer for Dalton Transactions
We often invoke the analogy of using building blocks when we talk about constructing large, complex molecules. Adding small building blocks often allows for finer control in design, and diatomic hydrogen is the most used small building block.
To add hydrogen to other molecules, hydrogen molecules themselves are split into a proton and hydride; these are then transferred to the target molecule. Recently, main group compounds involving frustrated Lewis pairs have been used in both steps instead of transition metal catalysts. There are also a few main group molecules where hydrogen is split at a single atomic site. Silylenes – the silicon analogue of carbenes, and the subject of this paper, with two bonds, a pair of electrons, and an empty p-orbital, are one type of these select few. This paper mentions the argument that non-transition metal catalysts may be “greener,” but a thorough life-cycle analysis of extraction, reaction and disposal would be necessary to indicate this.
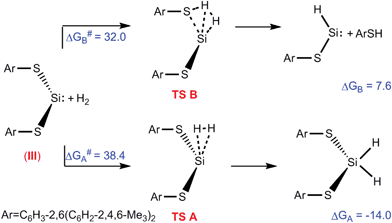
Authors, Kuriakose and Vanka use Density Functional Theory to explore the question of why some silylenes split hydrogen and some don’t. Specifically, they want to test the hypothesis that whether or not hydrogen is split at the silicon centre depends on if an adjacent atom “interferes,” leading to undesired products. They create a profile of the energies of reaction (with hydrogen gas) of three distinct types of silylene: a boryl amido silylene (I), a silyl amido silylene (II) and a dithiolate silylene (III). I and II have activated hydrogen, while III hasn’t been observed to do so.
The free energy profiles for the reaction of silylene, III, with hydrogen
After optimizing geometry they model the HOMO and LUMO of the silylenes, since donation of electrons from the HOMO and acceptance of electrons into the LUMO are necessary for hydrogen activation. In the case of I and II, the LUMO is localized on the silicon atom, while in III, it is not. Correspondingly, the reaction pathway of splitting hydrogen is energetically favoured for I and II, and disfavoured for III. They also examine effects of other features, such as the angle of the two substituents.
Have a read of the full article now:
New insights into small molecule activation by acyclic silylenes: a computational investigation
Nishamol Kuriakose and Kumar Vanka
Dalton Transactions, DOI: 10.1039/c3dt52817k
 Ian Mallov is currently a Ph.D. student in Professor Doug Stephan’s group at the University of Toronto. His research is focused on synthesizing new Lewis-acidic compounds active in Frustrated Lewis Pair chemistry. He grew up in Truro, Nova Scotia and graduated from Dalhousie University and the University of Ottawa, and worked in chemical analysis in industry for three years before returning to grad school.
Ian Mallov is currently a Ph.D. student in Professor Doug Stephan’s group at the University of Toronto. His research is focused on synthesizing new Lewis-acidic compounds active in Frustrated Lewis Pair chemistry. He grew up in Truro, Nova Scotia and graduated from Dalhousie University and the University of Ottawa, and worked in chemical analysis in industry for three years before returning to grad school.