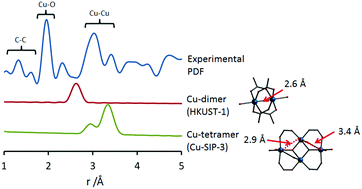
Ultrasound-driven preparation and pair distribution function-assisted structure solution of a copper-based layered coordination polymer
M. Infas Mohideen, Phoebe K. Allan, Karena W. Chapman, Joseph A. Hriljac and Russell E. Morris
Dalton Trans., 2014, Advance Article
DOI: 10.1039/C3DT53124D
Free to access until 17th January 2014
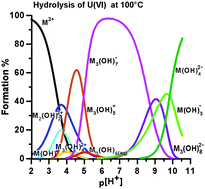
A calorimetric study of the hydrolysis and peroxide complex formation of the uranyl(VI) ion
Pier Luigi Zanonato, Plinio Di Bernardo and Ingmar Grenthe
Dalton Trans., 2014, Advance Article
DOI: 10.1039/C3DT52922C
Free to access until 17th January 2014
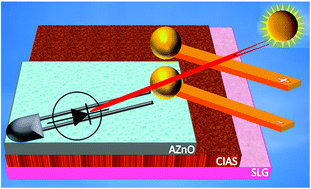
Transport properties of CuIn1-xAlxSe2/AZnO heterostructure for low cost thin film photovoltaics
Banavoth Murali and S. B. Krupanidhi
Dalton Trans., 2014, Advance Article
DOI: 10.1039/C3DT52515E
Free to access until 17th January 2014
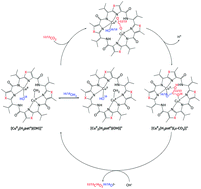
Carbonic anhydrase activity of dinuclear CuII complexes with patellamide model ligands
Peter Comba, Lawrence R. Gahan, Graeme R. Hanson, Marcel Maeder and Michael Westphal
Dalton Trans., 2014, Advance Article
DOI: 10.1039/C3DT53135J
Free to access until 17th January 2014
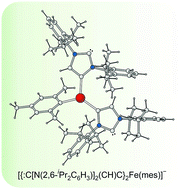
Iron (II) complexes of ditopic carbanionic carbenes
Rebecca A. Musgrave, Robert S. P. Turbervill, Mark Irwin, Radovan Herchel and Jose M. Goicoechea
Dalton Trans., 2014, Advance Article
DOI: 10.1039/C3DT52638K
Free to access until 3rd January 2014
Water-oxidation catalysis by synthetic manganese oxides – systematic variations of the calcium birnessite theme
Carolin E. Frey, Mathias Wiechen and Philipp Kurz
Dalton Trans., 2014, Advance Article
DOI: 10.1039/C3DT52604F
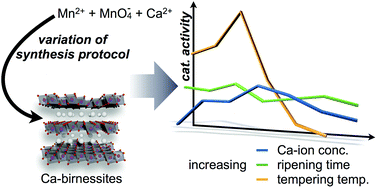
Free to access until 3rd January 2014
Ethyl 2-Hydroxy-2-methylpropanoate Derivatives of Magnesium and Zinc. The Effect of Chelation on the Homo- and Copolymerization of Lactide and e-Caprolactone.
Vagulejan Balasanthiran, Malcolm H. Chisholm, Kittisak Choojun and Christopher B. Durr
Dalton Trans., 2014, Advance Article
DOI: 10.1039/C3DT52553H
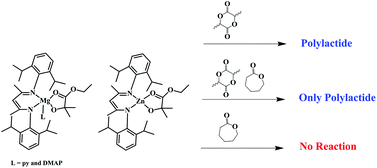
Free to access until 3rd January 2014
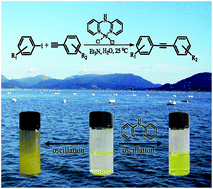
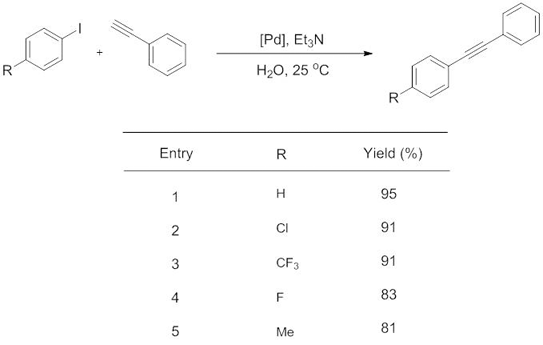
Copper-free Sonogashira Cross-coupling Reaction Promoted by Palladium complexes of Nitrogen-containing Chelating Ligand in Neat Water at Room Temperature
Hong Zhong, Jinyun Wang, Liuyi Li and Ruihu Wang
Dalton Trans., 2014, Advance Article
DOI: 10.1039/C3DT52970C
Free to access until 3rd January 2014












 Dr C. Liana Allen is currently a post-doctoral research associate in the group of Professor Scott Miller at Yale University, where she works on controlling the enantio- or regioselectivity of reactions using small peptide catalysts. Liana received her Ph.D. in organic chemistry at Bath University with Professor Jonathan Williams, where she worked on developing novel, efficient syntheses of amide bonds.
Dr C. Liana Allen is currently a post-doctoral research associate in the group of Professor Scott Miller at Yale University, where she works on controlling the enantio- or regioselectivity of reactions using small peptide catalysts. Liana received her Ph.D. in organic chemistry at Bath University with Professor Jonathan Williams, where she worked on developing novel, efficient syntheses of amide bonds.