Posted on behalf of Ian Mallov, web writer for Dalton Transactions
If you’ve ever donated blood you may recognize leishmaniasis as a disease on the questionnaire that may prevent you from doing so. Caused by parasites transmitted to humans by the bite of sandflies, British troops serving in the Middle East in World War I referred to it as “Jericho’s Buttons,” as many contracted the disease, with its characteristic button-shaped red skin lesions, near the ancient city of Jericho. Today new cases of leishmaniasis are estimated at 1.5-2 million worldwide per year, most in Latin America, the Indian sub-continent and the Middle-East. Leishmaniasis caused over 50,000 deaths worldwide in 2010.
The best-known cures for the disease are two drugs based, strangely, on antimony, like its periodic table neighbour arsenic known for forming compounds of extreme toxicity. These drugs – sodium stibogluconate (SSG) and meglumine antimoniate (MGA) – have been used for over seventy years. They must be administered by injection and closely monitored, and pockets of population have developed resistance to them. Though in the long-term a vaccine is hoped for, in the near term new or more easily-administered drugs are needed.
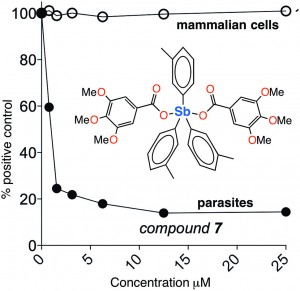
Andrews et. al. explore the curative potential – as well as the human toxicity – of twenty-six new compounds of antimony. These compounds were obviously designed to have some similar features to the existing SSG and MGA, but also to be simple. They feature two carboxylate groups, and three phenyl, benzyl, or ortho-, meta-, or para-tolyl groups bound to antimony. Many syntheses required only simple salt metathesis reactions between antimony(V)triorgano dibromides and sodium or potassium carboxylates at room temperature.
The researchers tested each in varying concentrations for toxicity on human primary fibroblast cells, and for anti-leishmanial activity on L. major promastigote parasites. As they state, the molecular mechanism by which SSG and MGA works in the body has not been confirmed, but is suspected to proceed via reduction of the antimony(V) species to an antimony(III) species by thiols. Their findings, though preliminary, show the trend that the meta- and para-tolylantimony molecules were the most effective against L. major promastigotes, while the ortho-tolyl derivatives were less effective, and those featuring phenyl or benzyl groups were more toxic to cells.
Their work certainly seems a useful step in establishing trends for designing new antimony drugs to combat leishmaniasis. One wonders, however, about issues of excretion of these drugs or their products from the human body – particularly in areas of the world whose water treatment lags behind First World standards.
Find out more from the paper:
Anti-leishmanial activity of heteroleptic organometallic Sb(V) compounds
uhammad Irshad Ali, Muhammad Khawar Rauf, Amin Badshah, Ish Kumar, Craig M. Forsyth, Peter C. Junk, Lukasz Kedzierski and Philip C. Andrews
Dalton Trans., 2013, Advance Article
DOI: 10.1039/C3DT51382C, Paper
 Ian Mallov is currently a Ph.D. student in Professor Doug Stephan’s group at the University of Toronto. His research is focused on synthesizing new Lewis-acidic compounds active in Frustrated Lewis Pair chemistry. He grew up in Truro, Nova Scotia and graduated from Dalhousie University and the University of Ottawa, and worked in chemical analysis in industry for three years before returning to grad school.
Ian Mallov is currently a Ph.D. student in Professor Doug Stephan’s group at the University of Toronto. His research is focused on synthesizing new Lewis-acidic compounds active in Frustrated Lewis Pair chemistry. He grew up in Truro, Nova Scotia and graduated from Dalhousie University and the University of Ottawa, and worked in chemical analysis in industry for three years before returning to grad school.