Posted on behalf of Lewis Downie, web writer for Dalton Transactions
Hg2+ is a potentially toxic ion which one would want to assess in drinking water and other potential sources of human consumption. It can have adverse effects on human health and negative consequences in the environment. Unfortunately, its detection is particularly difficult due to the electronic configuration of Hg2+ – this diamagnetic state leads to challenges when measuring by spectroscopic or magnetic means. In general chemosensors for such applications have to be complex. Recently, a simpler pyrene-pyrimidine based chemosensor has been reported for Hg2+.1
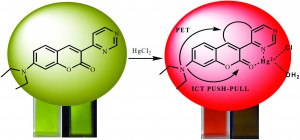
Inspired by this, the current work has linked a heterocyclic coumarin moiety to pyrimidine. In this molecule the electronic, and therefore chromophoric properties, are affected by binding to Hg2+. This change in colour – from green to red when bound – is induced by intramolecular charge transfer due to a strong “push-pull” interaction between the NEt2 group bound to the coumarin and the pyrimidine.
This colour change response appears to be selective for Hg2+ as no response is seen for Cu2+, Fe2+, Fe3+, Co2+, Ni2+, Pb2+, Zn2+, Cr3+, Mn2+ or Cd2+. The same selectivity is observed when more than one metal ion is present. It is suggested that the particular sensitivity for Hg2+ is mainly due to a size effect – the Hg2+ alone is capable of keeping the two aryl systems present in the same plane, which then allows the conjugation of the whole system beginning at the lone pair of the nitrogen on the NEt2 group found on the chromophore.
This system has a colour change which is visible to the naked eye and it is even possible to discern the concentration of the Hg2+ by eye. This property became apparent when paper was coated with the chemoselective material and a solution of a known concentration of Hg2+ led to different shades of orange; this shows promise as a simple and portable Hg2+ detector. It has also been found that the addition of KI to the reaction leads to the removal of Hg2+ from the chemoreceptor and a return to the green colour. Further addition of Hg2+, however, leads to the red colour being re-established. This continued addition of KI followed by Hg2+ can go on for a number of cycles with little change to the efficacy.
Find out more from the paper:
‘Pet’ vs. ‘Push-Pull’ induced ICT: A remarkable Coumarinyl-appended Pyrimidine based Naked Eye Colorimetric and Fluorimetric Sensor for detection of Hg2+ ion in aqueous media with test strips
Shyamaprosad Goswami, Avijit Kumar Das and Sibaprasad Maity
Dalton Trans., 2013, Accepted Manuscript
DOI: 10.1039/C3DT52252K, Communication
1. J. Weng, Q. Mei, Q. Ling, Q. Fan, W. Huang, Tetrahedron, 2012, 68, 14, 3129-3134
 Lewis Downie has wide ranging interests in the chemical sciences but has a background in functional materials. His main research focus is the investigation of these materials using crystallographic techniques. He is currently a postdoctoral research assistant at the University of St Andrews, U.K.
Lewis Downie has wide ranging interests in the chemical sciences but has a background in functional materials. His main research focus is the investigation of these materials using crystallographic techniques. He is currently a postdoctoral research assistant at the University of St Andrews, U.K.