 Scientists from Pacific Northwest National Laboratory have investigated the mechanism of heterolytic H2 activation by frustrated lewis pairs (FLPs). To do this, they employed an unique approach of solution calorimetry and were able to obtain the enthalpies and relative rates of H2 activation.
Scientists from Pacific Northwest National Laboratory have investigated the mechanism of heterolytic H2 activation by frustrated lewis pairs (FLPs). To do this, they employed an unique approach of solution calorimetry and were able to obtain the enthalpies and relative rates of H2 activation.
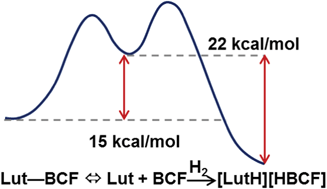
With the exception of previous studies that partly relied on theoretical calculations, there has not been a great deal of work devoted to understanding how molecular hydrogen is activated by FLPs. Autrey and colleagues have found that H2 activation rates using such species are relatively fast considering no metals are involved. They discovered that the rate was dependent on the structure of the Lewis basic amine involved, and that diffusion of hydrogen across the gas-liquid interface is not rate limiting.
Further work is needed to extract fundamental rate constants, kinetic orders, and activation barriers, say the researchers.
Read the article now to find out more…
A thermodynamic and kinetic study of the heterolytic activation of hydrogen by frustrated borane–amine Lewis pairs
Abhi Karkamkar, Kshitij Parab, Donald M. Camaioni, Doinita Neiner, Herman Cho, Thomas K. Nielsen and Tom Autrey
This article is part of an upcoming themed issue on Boranes and Borohydrides, guest edited by Simon Aldridge. Other articles by this author include:
Methods to stabilize and destabilize ammonium borohydride
Thomas K. Nielsen, Abhi Karkamkar, Mark Bowden, Flemming Besenbacher, Torben R. Jensen and Tom Autrey
Also of interest… Take a look at our recent themed issue in Frustrated Lewis Pairs










