Research in metal-organic frameworks (MOFs) or porous coordination polymers (PCPs) has exploded lately due to their potential applications in diverse areas from gas storage to drug delivery, but the Kitagawa group has been looking at yet another application that has rarely been considered: proton conductivity. This process would work in a similar way to Nafion, a DuPont product produced since the 1960s in which protons on SO3H groups hop between acid sites that extend from a Teflon backbone. Modified Nafion’s excellent mechanical and thermal stability allow it to be used as a proton conductor for proton exchange membrane (PEM) fuel cells.
 Foo et al. have synthesised a framework containing sulphonic acid groups, where sodium cations were exchanged in situ for protons. The resultant framework proved to be unstable to loss of guest molecules and, as such, its use in most future applications is limited.
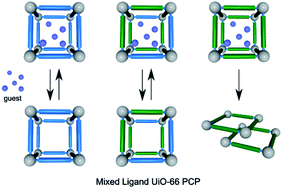
Foo et al. have synthesised a framework containing sulphonic acid groups, where sodium cations were exchanged in situ for protons. The resultant framework proved to be unstable to loss of guest molecules and, as such, its use in most future applications is limited.
However, through a solid solution approach, where a mixture of ligands was used in differing proportions, mixed MOFs were produced. The stand-out product was a framework in which 18% of the linkers contained sulphonic acid groups, which retained crystallinity and porosity following evacuation of guest molecules. The incorporation of this small proportion of acidic groups increased both the total uptake and the heat of adsorption of CO2 at 288 K.
Read about a solid solution approach as an alternative route to stabilising MOFs in this HOT article.
Ligand-based solid solution approach to stabilisation of sulphonic acid groups in porous coordination polymer Zr6O4(OH)4(BDC)6 (UiO-66)
Maw Lin Foo, Satoshi Horike, Tomohiro Fukushima, Yuh Hijikata, Yoshiki Kubota, Masaki Takata and Susumu Kitagawa










