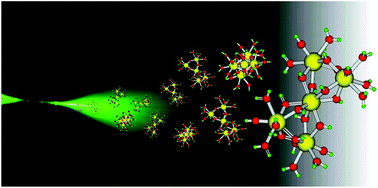
Although it has been known for some time that highly charged cations such as Th(IV) are susceptible to hydrolysis and subsequent polymerization, very little has been know about the exact nature of such polymeric species until now. Rothe et al. shed new light on this using a combination of X-ray absorption fine structure (XAFS) spectroscopy, high energy X-ray scattering (HEXS) measurements, and quantum chemical calculations to yield the most favourable structure as two Th(IV) dimers linked by a central Th(IV) cation through hydroxide bridges. This should have important implications in geology and waste reprocessing amongst others.
The solution structure of the Th(IV)-hydroxo pentamer
Read more for FREE for 4 weeks at:
Thorium nanochemistry: the solution structure of the Th(IV)–hydroxo pentamer
Clemens Walther, Jörg Rothe, Bernd Schimmelpfennig and Markus Fuss
Dalton Trans., 2012, Advance Article
DOI: 10.1039/C2DT30243H
Also of interest may be:
Infrared spectra and structures of the Th(OH)2 and Th(OH)4 molecules
Xuefeng Wang and Lester Andrews
Phys. Chem. Chem. Phys., 2005,7, 3834-3838
DOI: 10.1039/B509401A