 Alkali-Metal Mediated Metallation (AMMM) is a recent advance in C–H to C–metal exchange and it could be very useful synthetic chemistry community. AMMM involves the mixing of a typically powerful metallating reagent (e.g. an alkali-metal compound) with a weaker metallating reagent to make a single ligand-shared molecular compound which seemingly displays the reactivity of the alkali-metal coupled with the selectivity and functional group tolerance of the subordinate metal. Hey Presto – a great metallation!
Alkali-Metal Mediated Metallation (AMMM) is a recent advance in C–H to C–metal exchange and it could be very useful synthetic chemistry community. AMMM involves the mixing of a typically powerful metallating reagent (e.g. an alkali-metal compound) with a weaker metallating reagent to make a single ligand-shared molecular compound which seemingly displays the reactivity of the alkali-metal coupled with the selectivity and functional group tolerance of the subordinate metal. Hey Presto – a great metallation!
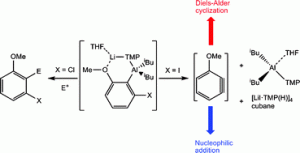
AMMM can be carried out in relatively cheap, non-polar solvents without the need for high temperatures. Usually Mg and Zn reagents are used in AMMM but recently +3 oxidation state aluminium reagents have proved effective. In this Hot Article, Robert Mulvey, Stuart Robertson and their team from Strathclyde have examined AMMM reactions with Al and studied the after effects of lithium-mediated alumination of 3-iodoanisole. To find out what they discovered read the article itself:
After-effects of lithium-mediated alumination of 3-iodoanisole: isolation of molecular salt elimination and trapped-benzyne products
Elaine Crosbie, Alan R. Kennedy, Robert E. Mulvey and Stuart D. Robertson
Dalton Trans., 2012, DOI: 10.1039/C2DT11893A










