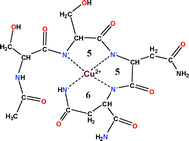
In this HOT article, Kállay and co-workers report on the synthesis and study of the copper complexes of rIAPP fragments. First of all the terminally protected tridecapeptide rIAPP(17–29) was investigated in which the potentially metal binding amino acid residues are located in the N-terminus. The surprisingly high affinity of this peptide towards complexation with copper(II) promoted a systematic study on the shorter fragments including rIAPP(17–22) (Ac-VRSSNN-NH2), rIAPP(17–20) (Ac-VRSS-NH2), its mutant peptides (Ac-VASSNH2 and Ac-VRAA-NH2) and rIAPP(19–22) (Ac-SSNN-NH2). The peptides can be classified into two different categories: (i) the tetrapeptides Ac-VRSS-NH2, Ac-VASS-NH2 and Ac-VRAA-NH2 can interact with copper(II) only under strongly alkaline conditions (pH > 10.0) and the formation of only one species with four amide nitrogen coordination can be detected; (ii) the peptides Ac-VRSSNNLGPVLPP-NH2, Ac-VRSSNN-NH2 and Ac-SSNN-NH2 can form complexes above pH 6.0 with the major stoichiometries [CuH−2L], [CuH−3L]− and [CuH−4L]2−. The data supports the idea that rIAPP(17–29) can interact with copper(II) ions under physiological conditions and the SSNN tetrapeptide fragment can be considered as the shortest sequence responsible for metal binding.
Read more for FREE until 21st September at:
Copper(II) complexes of rat amylin fragments
Csilla Kállay, Ágnes Dávid, Sarolta Timári, Eszter Márta Nagy, Daniele Sanna, Eugenio Garribba, Giovanni Micera, Paolo De Bona, Giuseppe Pappalardo, Enrico Rizzarelli and Imre Sóvágó
Dalton Trans., 2011, Advance Article
DOI: 10.1039/C1DT10835B










