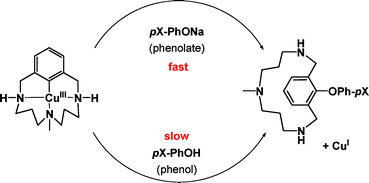
 In this HOT article, Ribas and co-workers build on previous studies on the mechanism of Ullmann cross coupling reactions using a well-defined aryl-copper(III) complex. Fundamental mechanistic knowledge of these couplings is still scarce but is gaining renewed interest due to the cost and toxicity benefits in comparison to Pd-based methodologies for the synthesis of key intermediates in the pharmaceutical industry. The reactivity of well defined aryl–CuIII species in front of phenol-type nucleophiles was found to differ substantially from the reactivity with corresponding phenolates, and a significant enhancement was found to produce the same aryl–O coupling product. Mechanistic studies showed that easy deprotonation of coordinated secondary amines was responsible for the intense LMCT band at 545 nm; indeed, this pH-dependent reactivity of the pincer-like coordinated ligand somewhat enhanced its reactivity.
In this HOT article, Ribas and co-workers build on previous studies on the mechanism of Ullmann cross coupling reactions using a well-defined aryl-copper(III) complex. Fundamental mechanistic knowledge of these couplings is still scarce but is gaining renewed interest due to the cost and toxicity benefits in comparison to Pd-based methodologies for the synthesis of key intermediates in the pharmaceutical industry. The reactivity of well defined aryl–CuIII species in front of phenol-type nucleophiles was found to differ substantially from the reactivity with corresponding phenolates, and a significant enhancement was found to produce the same aryl–O coupling product. Mechanistic studies showed that easy deprotonation of coordinated secondary amines was responsible for the intense LMCT band at 545 nm; indeed, this pH-dependent reactivity of the pincer-like coordinated ligand somewhat enhanced its reactivity.
Further catalysis reading available for FREE at:
Aryl–O reductive elimination from reaction of well-defined aryl–CuIII species with phenolates: the importance of ligand reactivity
Alicia Casitas, Nikolaos Ioannidis, George Mitrikas, Miquel Costas and Xavi Ribas
Dalton Trans., 2011, Advance Article
DOI: 10.1039/C1DT10428D











