The BBC radio series Discovery – Can Chemistry Save the World? presents an episode on Green Chemistry , well worth a listen:
, well worth a listen:
Discovery – Can Chemistry Save the World?
Hot Article: Photocatalytic dechlorination of chlorobenzene
In this Catalysis Science & Technology Hot Article, Hiroshi Kominami and colleagues examined the photocatalytic dechlorination of chlorobenzene.
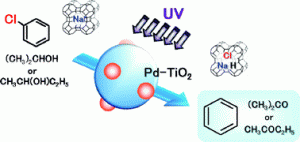 The dechlorination reaction, carried out in a 2-propanol suspension of titanium oxide powder in the presence of NaOH with palladium co-catalyst, resulted in almost quantitative formation of benzene and acetone in a very short time.
The dechlorination reaction, carried out in a 2-propanol suspension of titanium oxide powder in the presence of NaOH with palladium co-catalyst, resulted in almost quantitative formation of benzene and acetone in a very short time.
The reaction could be applicable to the recovery of useful aromatic compounds from chlorinated organic waste.
Read the article for free!
Stoichiometric formation of benzene and ketones by photocatalytic dechlorination of chlorobenzene in secondary alcohol suspensions of palladium-loaded titanium(IV) oxide powder in the presence of sodium ion sources
Kojirou Fuku, Keiji Hashimoto and Hiroshi Kominami
Catal. Sci. Technol., 2011, Advance Article
DOI: 10.1039/c0cy00040j, Paper
Hot Article: TAPping into adsorption to improve catalysis
All TAP micro-reactors have inert particles which are used so that the catalyst zone can be maintained under isothermal conditions. Even on “inert” particles adsorption will occur to some degree; however, the extent to which this occurs has a critical influence on the analysis of the TAP data.
In their recent paper, Chris Hardacre and co-workers, from Queen’s University, Belfast, UK, discuss the development of a function which accounts for the adsorption over the inert material, so that the TAP data analysis can be accurately determined. They also demonstrate this analysis method using the selective reduction of oxygen in a hydrogen rich ethylene feed over silver catalysts as a case study. Find out how they got on by reading their Catalysis Science & Technology Hot Article.
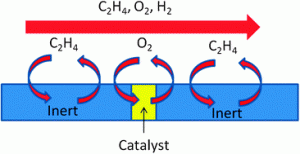 Correction for a possible reversible adsorption over an “inert” material
Correction for a possible reversible adsorption over an “inert” material
Alexandre Goguet, Christopher Hardacre, Burapat Inceesungvorn, Kevin Morgan and Sergiy O. Shekhtman
Catal. Sci. Technol., 2011, DOI: 10.1039/C0CY00075B, Paper
Challenge and progress: palladium-catalyzed sp3 C–H activation
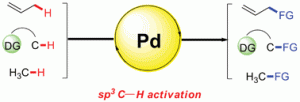 In the last several decades, direct sp2 C–H activation of (hetero)arenes as well as some olefins has been extensively investigated and found impressive applications in organic synthesis, which has been the subject of many papers and reviews. In comparison, much less research has been devoted to the activation of more ‘‘inert’’ sp3 C–H bonds of alkyl groups.
In the last several decades, direct sp2 C–H activation of (hetero)arenes as well as some olefins has been extensively investigated and found impressive applications in organic synthesis, which has been the subject of many papers and reviews. In comparison, much less research has been devoted to the activation of more ‘‘inert’’ sp3 C–H bonds of alkyl groups.In this Catalysis Science & Technology Perspective, Zhang-Jie Shi and colleagues tell us about the progress in the field using Palladium catalysis. Get quickly up to date in this area by reading their Perspective Article:
Hu Li, Bi-Jie Li and Zhang-Jie Shi
Catal. Sci. Technol., 2011, DOI: 10.1039/C0CY00076K, Perspective
Meet the Catalysis Science & Technology team this year!
We are attending a number of conferences this year and would be delighted to meet you there. The Catalysis Science & Technology team plan to attend:
06/03/2011, GRC: Inorganic Reaction Mechanisms, Galveston, TX, USA, Meet Ruth
27/03/2011, ACS National Meeting and Exposition, Anaheim, California, USA, Meet Jamie
11/04/2011, First EuCheMS Inorganic Chemistry Conference, Manchester, UK, Meet Jamie
19/04/2011, 17th Rideal Conference, Cardiff, UK, Meet Jamie
22/05/2011, CAMURE-8 International Symposium on Multifunctional Reactors, Naantali, Finland, Meet Ruth
05/06/2011, 22nd Meeting of the North American Catalysis Society, Detroit, MI USA, Meet Jamie
10/07/2011, GRC: Organometallic, Newport, RI, USA, Meet Ruth
03/07/2011, EuCOMC XIX, Toulouse, France, Meet Ruth
10/07/2011, 19th International Symposium on Olefin Metathesis and Related Chemistry, Rennes, France, Meet Jamie
07/08/2011, ICBIC, Vancouver, Canada, Meet Jamie
04/09/2011, EuCheMS Conference on Nitrogen Ligands , Granada, Spain, Meet Jamie
So as you can see we will be visiting a large number of conferences this year – let us know if you will be there too!

Ruth Doherty, Deputy Editor Catalysis Science & Technology
HOT Article: New catalysts for glutamic acid synthesis
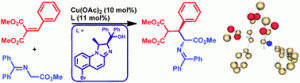 Wei-Ping Deng and colleagues from Shanghai in collaboration with John Fossey from the University of Birmingham have developed new chiral Cu complexes that catalyse the formation of 3-aryl glutamic acid derivatives in this Catalysis Science & Technology HOT Article.
Wei-Ping Deng and colleagues from Shanghai in collaboration with John Fossey from the University of Birmingham have developed new chiral Cu complexes that catalyse the formation of 3-aryl glutamic acid derivatives in this Catalysis Science & Technology HOT Article.
Glutamic acid and its derivatives are biologically important because they are essential components of peptides and proteins and also work as signal mediators.
The synthesis was carried out via catalytic asymmetric 1,4-addition reactions of glycine derivatives to
various alkylidene malonates with good yields and high stereoselectivities under the control of new N,O ligands.
Read more for free until 22nd March 2011
Novel N,O-Cu(OAc)2 complex catalysed diastereo- and enantioselective 1,4-addition of glycine derivatives to alkylidene malonates
Ming Wang, Yu-Hua Shi, Jun-Fei Luo, Wenting Du, Xiao-Xin Shi, John S. Fossey and Wei-Ping Deng
Catal. Sci. Technol, 2011, Advance Article
DOI: 10.1039/C0CY00001A, Paper
Hot Article: Saving energy in making cyclic carbonates
Process to make cyclic carbonates could now be done at room temperature and at atmospheric pressure
Bimetallic aluminium(acen) complexes are highly active catalysts for cyclic carbonate synthesis that can operate at atmospheric pressure and at room temperature. Cyclic carbonates are manufactured on a large scale from epoxides and carbon dioxide. They are used as degreasing agents, electrolytes for lithium-ion batteries and polar aprotic solvents, and can be converted into dimethyl carbonate – an oxygenating additive for petrol and aviation fuel. Current processes to make them use catalysts that need high temperatures and pressures to operate.
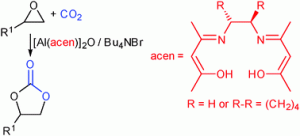 As featured in his Catalysis Science & Technology Hot Article, Michael North’s reaction requires a co-catalyst called tetrabutylammonium bromide, but the process can be carried out at room temperature and atmospheric pressure with a range of terminal epoxides.
As featured in his Catalysis Science & Technology Hot Article, Michael North’s reaction requires a co-catalyst called tetrabutylammonium bromide, but the process can be carried out at room temperature and atmospheric pressure with a range of terminal epoxides.
The global chemicals industry needs to develop alternative and sustainable starting materials. One solution is to use carbon dioxide as a starting material for the industrial synthesis of chemicals or fuels, but it is essential that such processes require little or no energy input or the dependence on fossil fuels will be restored. This leads to a requirement for reactions that can be carried out at atmospheric pressure and at or near room temperature.
Bimetallic aluminium(acen) complexes as catalysts for the synthesis of cyclic carbonates from carbon dioxide and epoxides
Michael North and Carl Young
Catal. Sci. Technol., 2011, Advance Article DOI: 10.1039/C0CY00023J, Paper
Hot Article: New cleaner catalysts for haloamines
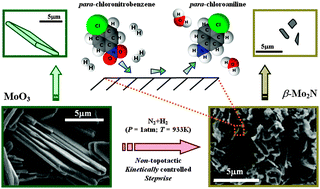
Mark Keane and co-workers have identified the intermediates in beta-Mo nitride synthesis and report the first application of this nitride in the catalytic hydrogenation of nitroarenes.
The selective hydrogenation of p-chloronitrobenzene (p-CNB) to p-chloroaniline (p-CAN) has been used as a model reaction.
The authors hope that their findings may serve as the basis for the development of Mo2N materials as new catalysts for the cleaner production of commercially important aromatic amines with multiple applications in the fine chemical industry.
Read more at:
Beta-Molybdenum nitride: synthesis mechanism and catalytic response in the gas phase hydrogenation of p-chloronitrobenzene
Fernando Cárdenas-Lizana, Santiago Gómez-Quero, Noémie Perret, Lioubov Kiwi-Minsker and Mark A. Keane
Catal. Sci. Technol., 2011, Advance Article
PERSPECTIVE: Near size zero attained
This PERSPECTIVE discuses the fundamentals and factors influencing the removal of the least reactive sterically hindered S-containing compounds present in transportation fuels, and more specifically in the diesel fraction. Special attention is paid to the progress made in alternative process concepts and technologies that are being developed for ultra low sulfur fuels.
Read more at:
Towards near zero-sulfur liquid fuels: a perspective review
Barbara Pawelec, Rufino M. Navarro, José Miguel Campos-Martin and José L. G. Fierro
Catal. Sci. Technol., 2011, Advance Article
DOI: 10.1039/C0CY00049C
PERSPECTIVE: Two-dimensional zeolites: dream or reality?
Zeolite synthesis, has been vigorously explored and developed for more than half a century and yet most zeolites known to date have not been synthesized without templating with an appropriate organic molecule. But the discovery of zeolite MCM-22 promises to change all that. The MCM-22 framework was found to form by two pathways and unlike conventional 3D zeolites the layered precursor could be structurally modified after synthesis. With 2D zeolites adsorption and catalysis occur almost exclusively on the surface of these materials and not inside the zeolite pores as in conventional zeolites.
This PERSPECTIVE aims to provide an up-to-date knowledge and challenges associated with “two-dimensional zeolites (2D zeolites)”.
Read more at:
Wieslaw J. Roth and Jiří Čejka, Catal. Sci. Technol., 2011, Advance Article DOI: 10.1039/C0CY00027B, Perspective