Bimetallic aluminium(acen) complexes are highly active catalysts for cyclic carbonate synthesis that can operate at atmospheric pressure and at room temperature. Cyclic carbonates are manufactured on a large scale from epoxides and carbon dioxide. They are used as degreasing agents, electrolytes for lithium-ion batteries and polar aprotic solvents, and can be converted into dimethyl carbonate – an oxygenating additive for petrol and aviation fuel. Current processes to make them use catalysts that need high temperatures and pressures to operate.
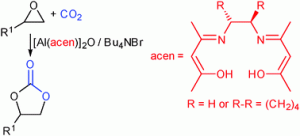 As featured in his Catalysis Science & Technology Hot Article, Michael North’s reaction requires a co-catalyst called tetrabutylammonium bromide, but the process can be carried out at room temperature and atmospheric pressure with a range of terminal epoxides.
As featured in his Catalysis Science & Technology Hot Article, Michael North’s reaction requires a co-catalyst called tetrabutylammonium bromide, but the process can be carried out at room temperature and atmospheric pressure with a range of terminal epoxides.
The global chemicals industry needs to develop alternative and sustainable starting materials. One solution is to use carbon dioxide as a starting material for the industrial synthesis of chemicals or fuels, but it is essential that such processes require little or no energy input or the dependence on fossil fuels will be restored. This leads to a requirement for reactions that can be carried out at atmospheric pressure and at or near room temperature.
Bimetallic aluminium(acen) complexes as catalysts for the synthesis of cyclic carbonates from carbon dioxide and epoxides
Michael North and Carl Young
Catal. Sci. Technol., 2011, Advance Article DOI: 10.1039/C0CY00023J, Paper










