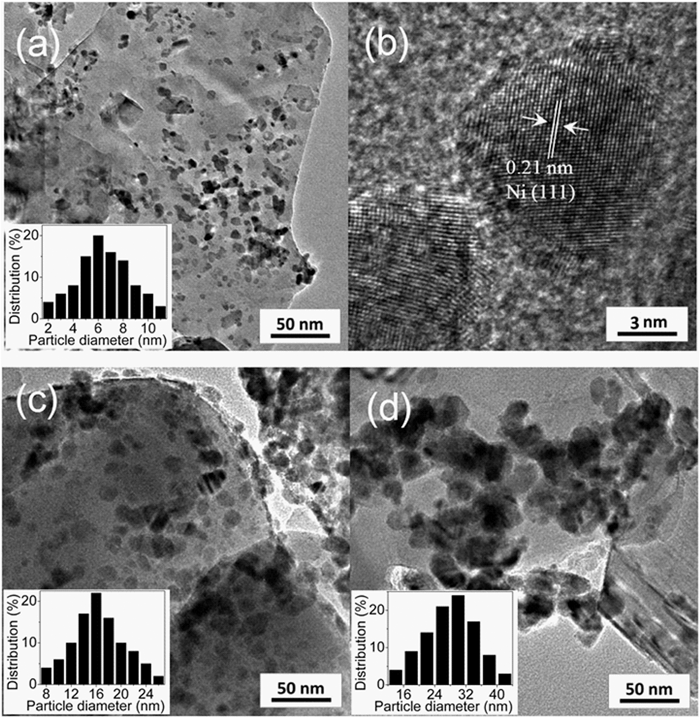
Catalytic decomposition of ammonium dinitramide (ADN) as high energetic material over CuO-based catalysts
Rachid Amrousse, Kohji Fujisato, Hiroto Habu, Ahmed Bachar, Claudine Follet-Houttemane and Keiichi Hori
Catal. Sci. Technol., 2013, Advance Article
DOI: 10.1039/C3CY00214D, Paper
A silver-free system for the direct C–H auration of arenes and heteroarenes from gold chloride complexes
Nanna Ahlsten, Gregory J. P. Perry, Xacobe C. Cambeiro, Tanya C. Boorman and Igor Larrosa
Catal. Sci. Technol., 2013, Advance Article
DOI: 10.1039/C3CY00240C, Communication
Highly practical iron-catalyzed C–O cleavage reactions
Dominik Gärtner, Hannelore Konnerth and Axel Jacobi von Wangelin
Catal. Sci. Technol., 2013, Advance Article
DOI: 10.1039/C3CY00266G, Communication
Tandem ethylene oligomerisation and Friedel–Crafts alkylation of toluene catalysed by bis-(3,5-dimethylpyrazol-1-ylmethyl)benzene nickel(II) complexes and ethylaluminium dichloride
Asheena Budhai, Bernard Omondi, Stephen O. Ojwach, Collins Obuah, Emmanuel Y. Osei-Twum and James Darkwa
Catal. Sci. Technol., 2013, Advance Article
DOI: 10.1039/C3CY00334E, Paper
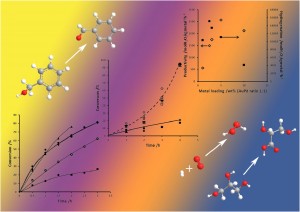
Evaluation of nanostructured vanadium(V) oxide in catalytic oxidations
Eric T. Drew, Yang Yang, Julia A. Russo, McKenzie L. Campbell, Samuel A. Rackley, JoAn Hudson, Patrik Schmuki and Daniel C. Whitehead
Catal. Sci. Technol., 2013, Advance Article
DOI: 10.1039/C3CY00183K, Paper