Sara Coles is a guest web-writer for Catalysis Science & Technology. She currently works for Johnson Matthey in Royston, UK.
The 20th EuCheMS Conference on Organometallic Chemistry was held from 30th June–4th July 2013, and co-sponsored by the RSC’s Catalysis Science & Technology and Dalton Transactions. This was the main international European conference on organometallic chemistry for 2013 and attendees came from all over the UK, Europe and further afield (notably the USA, Japan and various African countries).
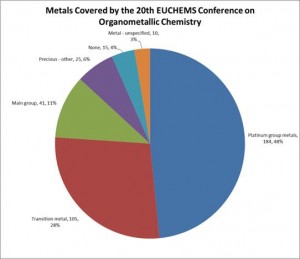
Palladium and ruthenium were by far the most represented metals. The presenters were split between those primarily studying fundamentals such as mechanism or characterisation techniques, and those with a focus on practical applications. Several were sponsored or supported by industrial or commercial companies, and others were clearly interested in developing commercialisable products. During the poster sessions, it was clear that some of the students were thinking about industry either in terms of their careers or of commercialising the products that they were working on.
 Popular catalytic themes included synthesis of novel compounds and complexes; the improvement of existing routes (including some current industrial processes); and waste reduction. Several presenters mentioned valorisation of waste biomass, a hot topic in the current climate. The cost of catalysts came up a few times. Often, though, the cost of the catalytic metal is secondary to other factors such as the cost of the ligand or of ultrapure solvents or reagents that must be added. It does not always follow that an expensive metal means a more expensive process – in fact the reverse is often the case.
Popular catalytic themes included synthesis of novel compounds and complexes; the improvement of existing routes (including some current industrial processes); and waste reduction. Several presenters mentioned valorisation of waste biomass, a hot topic in the current climate. The cost of catalysts came up a few times. Often, though, the cost of the catalytic metal is secondary to other factors such as the cost of the ligand or of ultrapure solvents or reagents that must be added. It does not always follow that an expensive metal means a more expensive process – in fact the reverse is often the case.
The conference was well attended and the main lecture theatre was full for the final presentation (by Bruno Chaudret, Institut National des Sciences Appliquées, Toulouse, France) at the end of day three. There was standing room only and the mood was exuberant after an inspiring three days.
There was an unscheduled additional presentation for Professor David Cole-Hamilton, of the University of St Andrews, in celebration of his long career in chemistry. The RSC prepared a special issue of Dalton Transactions, co-ordinated by Professors Duncan Bruce and Derek Woollins. Poster prizes were judged by the Young Plenary lecturers and were presented by Professor Dr Eric Meggers. There were nine winners and the prizes were books on organometallic chemistry contributed by Springer and the RSC.
The social activities included a musical interlude following Professor Ei-ichi Negishi’s presentation on Sunday evening. This was followed by a whisky tasting on Monday and to finish off the conference in style on Wednesday evening, a banquet and Scottish ceilidh were held in the glorious Scottish sunshine in the famous Old Course Hotel of St Andrews.
For those who were able to stay, there was an additional morning of chemistry at the RSC Awards Symposium on Thursday 4th July.
The 21st EuCheMS meeting will be held in Bratislava, Slovakia, from 5th–9th July 2015 and is being co-organised by the Czech and Slovak chemistry societies. The website is www.eucomcxxi.eu and will contain more information shortly.
Read some papers by speakers at the event in Catalysis Science & Technology:
Amination and dehydration of 1,3-propanediol by hydrogen transfer: reactions of a bio-renewable platform chemical
Sophie D. Lacroix, Annie Pennycook, Shifang Liu, Thomas T. Eisenhart and Andrew C. Marr
Catal. Sci. Technol., 2012, 2, 288-290, DOI: 10.1039/C1CY00339A
Organometallic hydrogen transfer and dehydrogenation catalysts for the conversion of bio-renewable alcohols
Andrew C. Marr
Catal. Sci. Technol., 2012, 2, 279-287, DOI: 10.1039/C1CY00338K
Direct coupling of alcohols to form esters and amides with evolution of H2 using in situ formed ruthenium catalysts
Martin H. G. Prechtl, Kathrin Wobser, Nils Theyssen, Yehoshoa Ben-David, David Milstein and Walter Leitner
Catal. Sci. Technol., 2012, 2, 2039-2042, DOI: 10.1039/C2CY20429K
NOBIN-based phosphoramidite and phosphorodiamidite ligands and their use in asymmetric nickel-catalysed hydrovinylation
Mike Schmitkamp, Walter Leitner and Giancarlo Franciò
Catal. Sci. Technol., 2013, 3, 589-594, DOI: 10.1039/C2CY20657A
Controlling selectivity in the reaction network of aldoxime hydrogenation to primary amines
Ewa Gebauer-Henke, Walter Leitner, Angelina Prokofieva, Henning Vogt and Thomas E. Müller
Catal. Sci. Technol., 2012, 2, 2539-2548, DOI: 10.1039/C2CY20356A
A latent ruthenium based olefin metathesis catalyst with a sterically demanding NHC ligand
Anita Leitgeb, Mudassar Abbas, Roland C. Fischer, Albert Poater, Luigi Cavallo and Christian Slugovc
Catal. Sci. Technol., 2012, 2, 1640-1643, DOI: 10.1039/C2CY20311A
A computational perspective of olefins metathesis catalyzed by N-heterocyclic carbene ruthenium (pre)catalysts
Raffaele Credendino, Albert Poater, Francesco Ragone and Luigi Cavallo
Catal. Sci. Technol., 2011, 1, 1287-1297, DOI: 10.1039/C1CY00052G
Gold(I)-catalyzed synthesis of furans and pyrroles via alkyne hydration
Pierrick Nun, Stéphanie Dupuy, Sylvain Gaillard, Albert Poater, Luigi Cavallo and Steven P. Nolan
Catal. Sci. Technol., 2011, 1, 58-61, DOI: 10.1039/C0CY00055H
Secondary phosphine oxides as pre-ligands for nanoparticle stabilization
Eoin Rafter, Torsten Gutmann, Florian Löw, Gerd Buntkowsky, Karine Philippot, Bruno Chaudret and Piet W. N. M. van Leeuwen
Catal. Sci. Technol., 2013,3, 595-599, DOI: 10.1039/C2CY20683H
NHC-stabilized ruthenium nanoparticles as new catalysts for the hydrogenation of aromatics
David Gonzalez-Galvez, Patricia Lara, Orestes Rivada-Wheelaghan, Salvador Conejero, Bruno Chaudret, Karine Philippot and Piet W. N. M. van Leeuwen
Catal. Sci. Technol., 2013,3, 99-105, DOI: 10.1039/C2CY20561K
Comments Off on An exuberant event: 20th Organometallic EuCheMS Conference
 Javier Pérez-Ramírez has been the Chair of Catalysis Engineering at the Institute for Chemical and Bioengineering, ETH Zurich since January 2010. Born and raised in Benidorm, Spain, Javier studied chemical engineering at the University of Alicante and later earned his PhD degree at the Delft University of Technology, The Netherlands in 2002. After spending some time in industry (2002-2005), holding several positions at Norsk Hydro and Yara International in Porsgrunn (Norway) where he was responsible of core projects related to catalyst development within fertilizer production, he was appointed ICREA research professor at ICIQ in Tarragona, Spain where he remained until his move to Zurich in 2010. The goal of this research is the discovery of energy-efficient chemical transformations that minimize byproducts, separation of waste and eliminate precious metals.
Javier Pérez-Ramírez has been the Chair of Catalysis Engineering at the Institute for Chemical and Bioengineering, ETH Zurich since January 2010. Born and raised in Benidorm, Spain, Javier studied chemical engineering at the University of Alicante and later earned his PhD degree at the Delft University of Technology, The Netherlands in 2002. After spending some time in industry (2002-2005), holding several positions at Norsk Hydro and Yara International in Porsgrunn (Norway) where he was responsible of core projects related to catalyst development within fertilizer production, he was appointed ICREA research professor at ICIQ in Tarragona, Spain where he remained until his move to Zurich in 2010. The goal of this research is the discovery of energy-efficient chemical transformations that minimize byproducts, separation of waste and eliminate precious metals.