Archive for the ‘Journal News’ Category
Professor Javier Pérez-Ramírez appointed as Associate Editor
Catalysis Science & Technology in C&EN
![]() A Graham Hutchings paper in Catalysis Science & Technology has been highlighted by C&EN, in their Science & Technology Concentrates.
A Graham Hutchings paper in Catalysis Science & Technology has been highlighted by C&EN, in their Science & Technology Concentrates.
You can find their news story midway down the Science & Technology section, entitled ‘Gold catalyzes Esterifications’: http://pubs.acs.org/cen/currentissue.html
The article is about Professor Hutchings work on ‘Oxidative esterification of 1,2-propanediol using gold and gold-palladium supported nanoparticles‘. In the paper they use gold and gold palladium nanoparticles to make methyl lactate (used in cosmetics) and methyl pyruvate (used to treat diseases of the nervous system).
Read Helen’s earlier blog to find out more: https://blogs.rsc.org/cy/2011/09/15/hot-article-nanoparticle-catalysis-with-the-midas-touch/
Don’t forget to follow us on Twitter, like us on Facebook and sign up for free access and table of content e-alerts.
Catalysis Science & Technology Issue 7
The cover of Cat alysis Science & Technology this month shows a small boy playing with various items such as spoons and forks with a mirror reflecting his play.
alysis Science & Technology this month shows a small boy playing with various items such as spoons and forks with a mirror reflecting his play.
The image was made by Kazuaki Ishihara and colleagues from Nagoya University, the Sekisui Medical Co., and the Japan Science and Technology Agency. The cover image illustrates the research from their Catalysis Science & Technology paper in Issue 7 on ‘Catalytic enantioselective alkyl and aryl addition to aldehydes and ketones with organozinc reagents derived from alkyl Grignard reagents or arylboronic acids’ Catal. Sci. Technol., 2011, 1, 1149-1158
More about Professor Ishihara’s work can be found on his webpage.
 The inside cover is by Liqiang Xu and co-workers from Shandong University, China and illustrates their work on ‘High yield synthesis of novel boron nitride submicro-boxes and their photocatalytic application under visible light irradiation’ Catal. Sci. Technol., 2011, 1, 1159-1165
The inside cover is by Liqiang Xu and co-workers from Shandong University, China and illustrates their work on ‘High yield synthesis of novel boron nitride submicro-boxes and their photocatalytic application under visible light irradiation’ Catal. Sci. Technol., 2011, 1, 1159-1165
See the full issue, which contains 2 perspectives, 7 communications and 16 full papers: Catalysis Science & Technology Issue 7
Did you see the cover of Issue 6? Read the explanation of the artwork by one of the authors in our earlier blog:
PERSPECTIVE: Imidazolium-derived organosilicas for catalysis
This perspective focuses o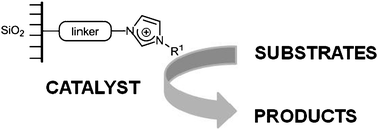
Read more for FREE about this growing area at:
Imidazolium-derived organosilicas for catalytic applications
Amàlia Monge-Marcet, Roser Pleixats, Xavier Cattoën and Michel Wong Chi Man
Catal. Sci. Technol., 2011, Advance Article
DOI: 10.1039/C1CY00287B
HOT Article: Nanoparticle catalysis with the Midas touch
Read the full article for FREE at:
Oxidative esterification of 1,2-propanediol using gold and gold-palladium supported nanoparticles
Gemma L. Brett, Peter J. Miedziak, Nikolaos Dimitratos, Jose A. Lopez-Sanchez, Nicholas F. Dummer, Ramchandra Tiruvalam, Christopher J. Kiely, David W. Knight, Stuart H. Taylor, David J. Morgan, Albert F. Carley and Graham J. Hutchings
Catal. Sci. Technol., 2011, Advance Article
DOI: 10.1039/C1CY00254F
Boron nitride nanosheet supports in catalysis
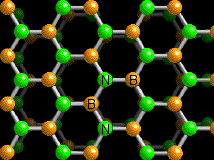 In the field of heterogeneous catalysis, the characteristics of the support can make a substantial difference in the operating conditions of a system and on its performance. Boron nitride (BN), in its hexagonal, graphite-like morphology, has proven to be a promising support for a number of catalysts and processes, including the synthesis of ammonia over barium/ruthenium catalysts thanks also to its higher chemical and thermal stability.
In the field of heterogeneous catalysis, the characteristics of the support can make a substantial difference in the operating conditions of a system and on its performance. Boron nitride (BN), in its hexagonal, graphite-like morphology, has proven to be a promising support for a number of catalysts and processes, including the synthesis of ammonia over barium/ruthenium catalysts thanks also to its higher chemical and thermal stability.
Chinese researchers led by Liqiang Xu focused on the synthesis of such supports, developing ultrathin nanosheets (<20 layers) with high specific surface area that could potentially be complementary to their carbon counterparts.
The group developed a convenient solid phase synthesis starting from boron oxide, zinc powder and N2H4 2HCl that yielded nanosheets with thickness varying from 2 to 6 nm. Notably, when zinc was absent, no BN was produced. Replacing zinc with iron or manganese resulted in lower yields or thicker nanosheets.
To test the properties of the new boron nitride support, the nanosheets were impregnated with platinum and employed in the oxidation of carbon monoxide with interesting results. Not only did the system reached almost full conversion with catalyst concentration as low as 2, 1 and 0.04%, but the respective temperature at which it was achieved (respectively 165, 210 and 310 °C) was lower than with conventional BN or alumina supports.
The material was also functionalised with gold nanoparticles, but to obtain interactions strong enough to anchor them to the surface, a supplementary functionalisation of the material was required. After hydrothermal treatment of the nanosheet with hydrogen peroxide, the Au nanoparticles dispersed more efficiently and allowed for higher loading.
In the Authors` view, these Au/BN nanosheet could find applications in optical materials, sensors and photocatalysis.
Find the full communication here.
Convenient synthesis and applications of gram scale boron nitride nanosheets
Liancheng Wang, Changhui Sun, Liqiang Xu and Yitai Qian
Catal. Sci. Technol., 2011, Advance Article
DOI: 10.1039/C1CY00191D
Catalysis Science & Technology is now on LinkedIn
 If you’re on LinkedIn you’ll be delighted to hear that we are too!
If you’re on LinkedIn you’ll be delighted to hear that we are too!
Catalysis Science & Technology now have our own LinkedIn group, so come join us, to keep in touch with what is going on in the journal.
.
LinkedIn not your thing? Sign up to get table of contents e-alerts instead. You get an email every month with a list of articles in each issue of Catalysis Science & Technology, and as all our content is free throughout 2011 and 2012, you’ll get to click through and read anything that catches your eye.
Ad hoc Titanium mesh for air and water purification
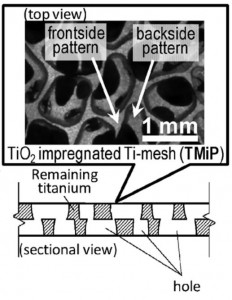 Titanium doxide is an extremely versatile photocatalyst that can be employed in a wide range of reactions used in environmental decontamination, such as, CO2 and nitric oxide oxidation among many. Not surprisingly new ways to employ this flexible system are developed every year.
Titanium doxide is an extremely versatile photocatalyst that can be employed in a wide range of reactions used in environmental decontamination, such as, CO2 and nitric oxide oxidation among many. Not surprisingly new ways to employ this flexible system are developed every year.
Recently, a large amount of research in this field focused on improving the characteristics of the support used for the catalyst, with the aim to enhance its performances or adapt to specific reaction conditions.
In this same context, the group led by Tsuyoshi Ochiai developed a procedure to easily fabricate catalytic filter material based on chemically etched titanium sheets impregnated with titanium dioxide for applications in air- and water-decontamination.
This new titanium mesh was fabricated by applying a layer of resist on both sides of a titanium sheet followed by chemical etching. The pattern of the pores could easily be controlled during the application of the resist layer, allowing to great control in the mesh structure. The finished structure was further modified to be finally coated with TiO2 anatase sol-gel.
The material was then tested against commercial titanium mesh coated following the same procedure in the photocatalytic (UV light promoted) degradation of acetaldehyde at room temperature and atmospheric pressure, revealing the superior performances of the new material, the measured rate constant of the group`s titanium mesh being 2.5 times higher than the commercial one.
The activity of the material was also assessed in water and determined with a mehylene blue decolorisation test. The purification performance both in air and water did not decrease over repeated tests, indicating a good adhesion of the TiO2 nanoparticles onto the surface of the mesh. Tests performed without irradiation resulted in no reaction, proving the photocatalytic nature of the process.
The increased activity of the new coated titanium mesh has been ascribed by the authors to the good morphology of their material, with its highly ordered structure of interconnected macropores and the crystallinity of the TiO2 coating.
Continue reading this communication here or access the ESI here.
Fabrication of a TiO2 nanoparticles impregnated titanium mesh filter and its application for environmental purification
Tsuyoshi Ochiai, Toru Hoshi, Houda Slimen, Kazuya Nakata, Taketoshi Murakami, Hiro Tatejima, Yoshihiro Koide, Ammar Houas, Takuji Horie, Yuko Morito and Akira Fujishima
Catal. Sci. Technol., 2011, Advance Article
DOI: 10.1039/C1CY00185J, Communication
Effective benzylic alcohol oxidation with simple catalysts
 The oxidation of alcohols to carbonyl compounds holds an important place in the transformations of organic molecules, especially the selective oxidation to aldehydes or ketones without overoxidation. The importance of these transformations lies in the usefulness and reactivity of carbonyl compounds as synthons in organic synthesis. Many techniques and reagents are available for this reaction, but they tend to suffer from the need for stoichometric amounts of oxidants, as in the case of permanganate and chromate based oxidants. Ideally, the most environmentally friendly and efficient process of transforming alchools in their respective carbonyl compounds would be by employing metal catalysts that can exploit molecular oxygen as the oxidant. Although several systems have been developed that achieve this goal, they generally require additives, ligands, sacrificial substrates or high pressures of oxygen.
The oxidation of alcohols to carbonyl compounds holds an important place in the transformations of organic molecules, especially the selective oxidation to aldehydes or ketones without overoxidation. The importance of these transformations lies in the usefulness and reactivity of carbonyl compounds as synthons in organic synthesis. Many techniques and reagents are available for this reaction, but they tend to suffer from the need for stoichometric amounts of oxidants, as in the case of permanganate and chromate based oxidants. Ideally, the most environmentally friendly and efficient process of transforming alchools in their respective carbonyl compounds would be by employing metal catalysts that can exploit molecular oxygen as the oxidant. Although several systems have been developed that achieve this goal, they generally require additives, ligands, sacrificial substrates or high pressures of oxygen.
With these limitations in mind, Ma and Lei have been focusing their efforts on the development of simpler systems to achieve selective oxidations of alcohols to aldehydes and ketons (a short selection of their recent publications can be found here and here) , and recently discovered a simple methodology that only requires AlBr3 6H2O and athmospheric oxygen to efficiently convert primary benzylic alcohols in their corresponding aldehydes with high selectivity and yields.
Employing a catalyst loading of 30% in dioxane in a batch reactor at the mild temperature of 70 °C afforded conversions of primary benzylic alcohols up to 100% with total selectivity for aldehyde in several cases, with 89% being the minimum selectivity measured. Running the reaction in an argon athmosphere instead of air resulted in only trace amounts of benzaldehydes and the use of anhydrous conditions did not change the outcomes of the transformation, suggesting that moisture in solvents or gases does not affect the reaction; in addition, nitro and ether moyeties on the ring were well tolerated.
In order to gather insights into the mechanism of the reaction, a set of isotope-labelling experiments have been performed and suggested that the oxygen atom is derived from the oxidant and not from a rearrangement of the original molecule.
When the conditions were applied to secondary benzylic alcohols, the conversion rate of several diphenylmethanols into the corrisponding benzophenones suroassed 94% with selectivities up to 80%, with the main side-products being the brominated derivatives.
In summary, Ma and Lei developed a simple system that does not use chlorinated solvents, environmentally unfriendly metals or complex ligands and conditions but still provided good yields and conversions with the substrates tested. Further study on a broader substrate scope, refinement of conditions and mechanisms are underway.
Read this interesting communication in Catalysis Science & Technology.
AlBr3·6H2O catalyzed oxidation of benzylic alcohols
Yun-Mei Zhong, Heng-Chang Ma, Jin-Xia Wang, Xiao-Jie Jia, Wen-Feng Li and Zi-Qiang Lei
Catal. Sci. Technol., 2011, Advance Article DOI: 10.1039/C1CY00165E, Communication
Strategies in organocatalysts immobilisation
The beginning of the new century, in chemistry, has been often linked to the renaissance of organocatalysis as enthusiastically reported by articles appearing on Angewandte Chemie and Chemical Society Reviews in the past few years. Although presenting several advantages over traditional metal-based catalysts, such as high stereoselectivity, ease of synthesis and handling, and the general feeling of being more environmentally benign, they yet have to persuade industry into their large scale application.
Some of the reasons behind the fall-out of industry for organocatalysts lie in the lower efficiency they exhibit, the major difficulties of catalyst separation, recovery and recycling. Attempts to covalently bind organocatalysts on polymeric and other supports addressed the problems of separation but often resulted in a loss of activity triggered by modifications of structure of the catalyst.
 In an interesting perspective appeared on Cat. Sci. Technol., Luo, Zhang and Chen presented an overview of alternative immobilisation techniques based on non-covalent bonds.
In an interesting perspective appeared on Cat. Sci. Technol., Luo, Zhang and Chen presented an overview of alternative immobilisation techniques based on non-covalent bonds.
As stated by the authors, the loss of activity on covalently bound organocatalysts is likely to depend on the changes in the structure and chemical properties that these small organic molecules undergo when bound to the support. Non-covalent immobilisation, on the other hand, seems to circumvent this problem and, despite other shortcomings, might be a promising field of study.
Examples of acid-base immobilisation presented include the use of solid acid like polyoxometalates or polystyrene sulfonic acids as the support, used with chiral amines-based catalysts in the aldol reaction and Michael additions, where recovery of the catalyst could be achieved by precipitation with diethyl ether. Other immobilisation strategies presented are the incorporation of the organocatalyst into a phase transfer catalysts (PTC) and the immobilisation into clays. The latter is achieved on materials such as montmorillonite, which comprises negatively charged layers alternated with layers of Na+ species.Using a cation exchange process, molecules such as proline and proline derived structures could be immobilized in the interlayers and the resulting material successfully used in aldol reactions. Another family of supports is represented by ionic liquids, where an organocatalysts is used as the anionic partner in the structure, creating chiral ionic liquid s that had been used to catalyse Mannich-type and aza-Diels-Alder reactions. Other supports mentioned are self-supported gel-type organocatalysts, biphasic immobilisation and techniques based on hydrophobic interactions, using cyclodextrines as the supports.
Several detailed examples and references for each category, inviting the interested reader to further reading.
In the authors` words – the non-covalent immobilisation is anticipated to provide a viable solution to enhance the applications of organocatalysis with “practical” credentials.
Read the full article here
Perspective
Non-covalent immobilisation of asymmetric organocatalysts
Long Zhang, Sanzhong Luo and Jin-Pei Cheng
Catal. Sci. Technol., 2011, Advance Article
DOI: 10.1039/C1CY00029B











