Dr Antony Williams of the RSC and Dr John Shockcor from Waters will be speaking on:
Connecting Chemistry and Mass Spectrometry on the Internet – ChemSpider
Monday 31 January 2011
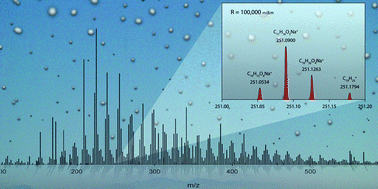
Connecting chemistry and mass spectrometry on the internet in the very first Chemistry World live webinar on 31 January, discover the powerful combination of the modern mass spectrometry and the ChemSpider database of chemical structures in metabolomics research.
Join the live webinar – Register Here
Or
Be part of the active audience at The Royal Society of Chemistry, London, UK – Register Here
This Chemistry World webinar is brought to you in partnership with ChemSpider and Waters.












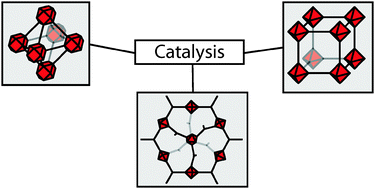 They categorise three classes of MOF catalysts:
They categorise three classes of MOF catalysts: PCCP Perspective article
PCCP Perspective article