Canadian scientists have, for the first time, been able to identify spectroscopically carbon dioxide clusters that could provide valuable information on intermolecular interactions.
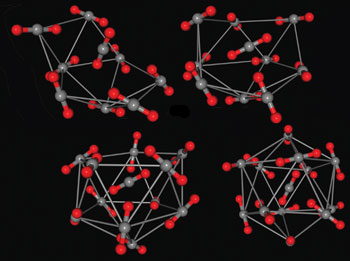
Despite the significance of carbon dioxide in atmospheric chemistry and use of supercritical carbon dioxide as an industrial solvent, the spectroscopic identification and study of carbon dioxide clusters have so far been limited to the dimer and trimer, as larger clusters can break up when being analysed.
Now, Robert McKellar and his team at the University of Calgary and the Steacie Institute for Molecular Sciences, Ontario, have identified (CO2)6 to (CO2)13 clusters using high resolution infrared (IR) spectroscopy, as published in their latest PCCP paper.
‘Studying clusters is a very good way of getting a handle on that because if we build up the cluster and measure its properties, then we’re really learning about the intermolecular forces in a direct way,‘ says McKellar.
Read the rest of the Chemistry World article by Yuandi Li…
Read the PCCP article:
Spectroscopic identification of carbon dioxide clusters: (CO2)6 to (CO2)13
J. Norooz Oliaee, M. Dehghany, N. Moazzen-Ahmadi and A. R. W. McKellar
Phys. Chem. Chem. Phys., 2011, 13, 1297-1300
Comments Off on Carbon dioxide clusters cracked by IR











 ‘HOT’ article
‘HOT’ article


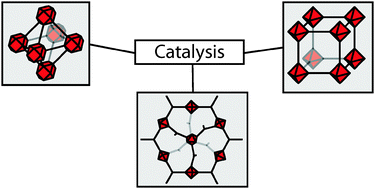 They categorise three classes of MOF catalysts:
They categorise three classes of MOF catalysts: