UK researchers can track the early steps of formation of peptide clumps linked to Alzheimer’s disease using the peptide’s fluorescent ability. This could help design effective therapies for the disease at an early stage.
A peptide known as beta-amyloid forms amyloid plaques that are found in Alzheimer’s disease. Scientists believe that the toxicity of the smallest peptide aggregates formed during the earliest stages of the aggregation process contribute to the neurological damage in the disease. But it’s difficult to study the earliest aggregation steps to find out why the peptide starts to clump together.
‘Fluorescence is sensitive to interactions on the Ångström to nanometre scale so it can be used to monitor processes between individual molecules at the early stages of amyloid aggregation,’ explains Rolinski.
The team measured the decay in tyrosine fluorescence at eight different peptide concentrations enabling them to detect early single peptide-peptide interactions, which are invisible in conventional fluorescence experiments. They found that the initial peptide concentration influences what conformation is adopted by individual peptides and determines the rate of their aggregation.
Read the rest of the Chemistry World story by Russell Johnson
Or read the PCCP paper in full:
Beta-amyloid oligomerisation monitored by intrinsic tyrosine fluorescence
Mariana Amaro, David J. S. Birch and Olaf J. Rolinski
Phys. Chem. Chem. Phys., 2011, DOI: 10.1039/c0cp02652b













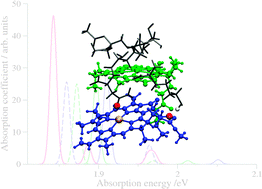 Subsystem TDDFT calculations allow us to investigate structural, environmental, and excitonic effects in optical spectra of (mutated) light-harvesting complexes.
Subsystem TDDFT calculations allow us to investigate structural, environmental, and excitonic effects in optical spectra of (mutated) light-harvesting complexes.
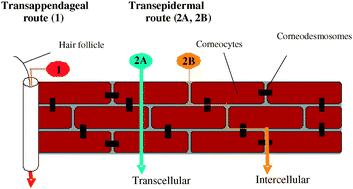 The authors explore the strategies currently employed to promote skin permeation and to consider the most exciting approaches currently under investigation. The limitations of current methodology to examine the problem are discussed. New opportunities to fill the gaps in our current knowledge are identified and the importance of interdisciplinary research in the field is emphasised.
The authors explore the strategies currently employed to promote skin permeation and to consider the most exciting approaches currently under investigation. The limitations of current methodology to examine the problem are discussed. New opportunities to fill the gaps in our current knowledge are identified and the importance of interdisciplinary research in the field is emphasised.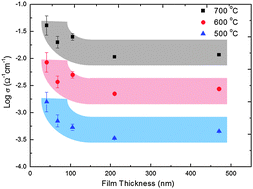 However, especially in the cases of the conductivity of ultra-thin films measured in an in-plane configuration, it is highly possible that the ‘apparent’ conductivity increase originates from electrical current flowing through other conduction paths than the thin film. As a systematic study to interrogate those measurement artifacts, we report various sources of electrical current leaks regarding in-plane conductivity measurements, specifically insulators in the measurement set-up.
However, especially in the cases of the conductivity of ultra-thin films measured in an in-plane configuration, it is highly possible that the ‘apparent’ conductivity increase originates from electrical current flowing through other conduction paths than the thin film. As a systematic study to interrogate those measurement artifacts, we report various sources of electrical current leaks regarding in-plane conductivity measurements, specifically insulators in the measurement set-up.