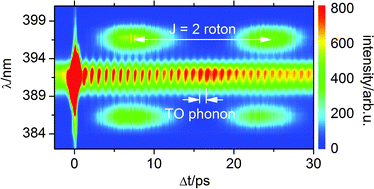
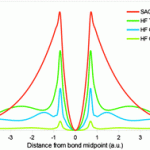
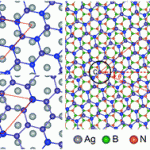
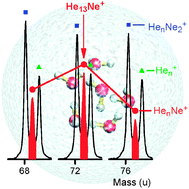
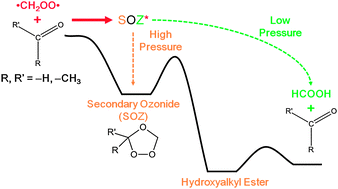
We are delighted to announce a cross journal web collection on porous materials in conjunction with the FEZA 2014 conference which will cover all aspects of science and technology associated with ordered porous materials – zeolites, zeotypes, mesostructured materials and porous coordination polymers.
Nine Royal Society of Chemistry publications are encouraging submissions for the collection:
– Catalysis Science & Technology
– Dalton Transactions
– Green Chemistry
– Journal of Materials Chemistry A
– Journal of Materials Chemistry B
– Journal of Materials Chemistry C
– Nanoscale
– Physical Chemistry Chemical Physics (PCCP)
– RSC Advances
The overall theme of the conference is “Porous Systems: From Novel Materials to Sustainable Solutions” and it will cover a wide range of topics from the synthesis of porous solids, advanced characterization, modeling, gas adsorption and separation, catalytic applications and natural occurring zeolites to the sustainable technological uses of porous systems and their applications in biology and medicine.
Submissions are welcome to the relevant journal across the themes of the conference. We encourage you to submit to this collection highlighting the challenges and opportunities in the fast moving field of porous materials.
Articles can be submitted from now until the 9th June 2014 and the collection will receive promotion at the conference in September. Please indicate in your covering letter that your submission is for consideration for the FEZA 2014 collection. Please note that all submissions will be subject to the normal peer review process.
If you have any queries or for more information, please contact the relevant Editorial Office: catalysis-rsc@rsc.org, dalton-rsc@rsc.org, materialsa-rsc@rsc.org, materialsb-rsc@rsc.org, materialsc-rsc@rsc.org, nanoscale-rsc@rsc.org, pccp-rsc@rsc.org or advances-rsc@rsc.org.











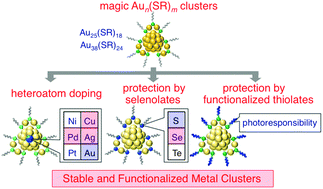
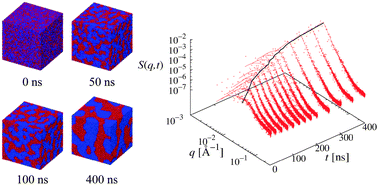

 We are saddened by the news that
We are saddened by the news that