We have had another record breaking year in 2010, with submissions continuing to rise, our biggest volume to date and our Impact Factor now leads the way with general physical chemistry journals.
 Please take a look at our New Year Editorial for 2011 and we invite you to submit some of your best work for publication in PCCP this year.
Please take a look at our New Year Editorial for 2011 and we invite you to submit some of your best work for publication in PCCP this year.
We thank all of our authors, Board members, readers and referees for their valuable support, it is you that has achieved this great success! We look forward to working with you in the future to build on our achievements.
Wishing you a Merry Christmas and all the best for the New Year!











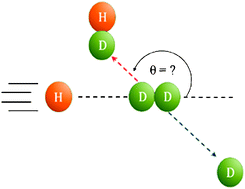 The hydrogen exchange reaction H + H2 -> H2 + H is the simplest neutral bimolecular reaction and for nearly a century has been a benchmark for studying reaction dynamics.
The hydrogen exchange reaction H + H2 -> H2 + H is the simplest neutral bimolecular reaction and for nearly a century has been a benchmark for studying reaction dynamics. Read the
Read the  Until now, research has only focussed on the catalysts’ active site and not on the reaction mechanisms. Graham Hutchings, Albert Carley and colleagues at the University of Cardiff have investigated the reaction mechanism occurring on a Au/Fe2O3 catalyst and found that CO dissociates at ambient temperature when co-adsorbed with O2.
Until now, research has only focussed on the catalysts’ active site and not on the reaction mechanisms. Graham Hutchings, Albert Carley and colleagues at the University of Cardiff have investigated the reaction mechanism occurring on a Au/Fe2O3 catalyst and found that CO dissociates at ambient temperature when co-adsorbed with O2. View the article on PhysOrg.com
View the article on PhysOrg.com Julian Eastoe and colleagues at the University of Bristol have discovered a new method to extract oil using CO2 in an efficient and environmentally friendly way.
Julian Eastoe and colleagues at the University of Bristol have discovered a new method to extract oil using CO2 in an efficient and environmentally friendly way.