PCCP is delighted to present its high profile themed issue on Materials innovation through interfacial physics and chemistry
 The issue is Guest Edited by Professor Katsuhiko Ariga, new PCCP Editorial Board member, and highlights some of the great work in this exciting area of chemistry.
The issue is Guest Edited by Professor Katsuhiko Ariga, new PCCP Editorial Board member, and highlights some of the great work in this exciting area of chemistry.
The two eye-catching covers feature the work of Frank Caruso and colleagues (DOI: 10.1039/C0CP02287J) and Song, Shelnutt et al. (DOI: 10.1039/C0CP01930E).
 The issue hosts an array of articles, including Communications, papers and these Perspective review articles:
The issue hosts an array of articles, including Communications, papers and these Perspective review articles:
Nanostructured polymer assemblies formed at interfaces: applications from immobilization and encapsulation to stimuli-responsive release
Yajun Wang, Leticia Hosta-Rigau, Hannah Lomas and Frank Caruso
Phys. Chem. Chem. Phys., 2011, 13, 4782
Operation of micro and molecular machines: a new concept with its origins in interface science
Katsuhiko Ariga, Shinsuke Ishihara, Hironori Izawa, Hong Xia and Jonathan P. Hill
Phys. Chem. Chem. Phys., 2011, 13, 4802
Nanoaggregate shapes at the air/water interface
D. Vollhardt, N. Nandi and S. Dutta Banik
Phys. Chem. Chem. Phys., 2011, 13, 4812











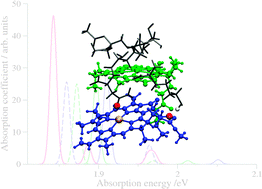 Subsystem TDDFT calculations allow us to investigate structural, environmental, and excitonic effects in optical spectra of (mutated) light-harvesting complexes.
Subsystem TDDFT calculations allow us to investigate structural, environmental, and excitonic effects in optical spectra of (mutated) light-harvesting complexes.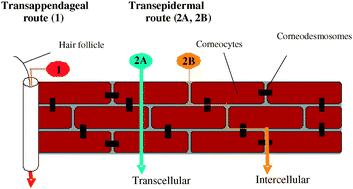 The authors explore the strategies currently employed to promote skin permeation and to consider the most exciting approaches currently under investigation. The limitations of current methodology to examine the problem are discussed. New opportunities to fill the gaps in our current knowledge are identified and the importance of interdisciplinary research in the field is emphasised.
The authors explore the strategies currently employed to promote skin permeation and to consider the most exciting approaches currently under investigation. The limitations of current methodology to examine the problem are discussed. New opportunities to fill the gaps in our current knowledge are identified and the importance of interdisciplinary research in the field is emphasised.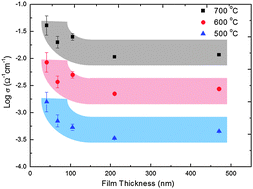 However, especially in the cases of the conductivity of ultra-thin films measured in an in-plane configuration, it is highly possible that the ‘apparent’ conductivity increase originates from electrical current flowing through other conduction paths than the thin film. As a systematic study to interrogate those measurement artifacts, we report various sources of electrical current leaks regarding in-plane conductivity measurements, specifically insulators in the measurement set-up.
However, especially in the cases of the conductivity of ultra-thin films measured in an in-plane configuration, it is highly possible that the ‘apparent’ conductivity increase originates from electrical current flowing through other conduction paths than the thin film. As a systematic study to interrogate those measurement artifacts, we report various sources of electrical current leaks regarding in-plane conductivity measurements, specifically insulators in the measurement set-up.


 ‘HOT’ article
‘HOT’ article