Experiments carried out by scientists in the US have provided new evidence in the controversial issue of surface freezing in alkane nanodroplets.

For small droplets, or systems with free surfaces, such as those in atmospheric aerosols, the freezing mechanism of hydrocarbons is a highly debated topic. Until now, experimental techniques have not been able to distinguish between surface and volume freezing.
Barbara Wyslouzil and colleagues at the Ohio State University, Columbus, are interested in understanding phase transitions and organization in nanodroplets. ‘Since surface-to-volume ratio increases as objects get smaller, nanodroplets present an obvious advantage for the study of surface effects,’ says Wyslouzil. Nanodroplets of n-octane and n-nonane were formed and rapidly cooled in a continuous flow supersonic Laval nozzle. As the condensable carrier gas mixture flows through the nozzle, the flow accelerates, effectively cooling as the pressure and temperature drop. This first condenses the vapour to liquid droplets, then freezes them.
Read the full article in Chemistry World here…
Read this article in PCCP:
Experimental evidence for surface freezing in supercooled n-alkane nanodroplets
Viraj P. Modak, Harshad Pathak, Mitchell Thayer, Sherwin J. Singer and Barbara E. Wyslouzil
DOI: 10.1039/C3CP44490B












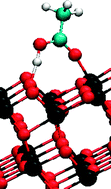 Sergei Manzhos and co-authors have calculated the anharmonic vibrations of the carboxyl group adsorbed on an anatase TiO2 surface in acetic acid. This is the first time vibrational spectra for different adsorption sites of an organic molecule have been computed and compared without neglecting anharmonicity and coupling of the attaching group. Their results are very valuable for the development of dye-sensitized solar cells because they identify the mode of adsorption of the dye on the semiconductor surface.
Sergei Manzhos and co-authors have calculated the anharmonic vibrations of the carboxyl group adsorbed on an anatase TiO2 surface in acetic acid. This is the first time vibrational spectra for different adsorption sites of an organic molecule have been computed and compared without neglecting anharmonicity and coupling of the attaching group. Their results are very valuable for the development of dye-sensitized solar cells because they identify the mode of adsorption of the dye on the semiconductor surface.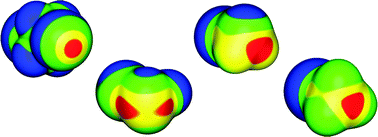
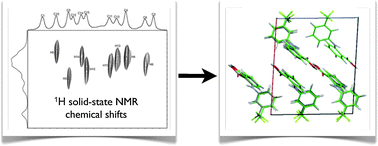 Structural characterisation of powdered solids remains a significant challenge in modern chemistry. Graeme Day, Lyndon Emsley and co-workers have used a combination of computational and solid state NMR approaches to determine the structures of powdered samples of small organic compounds.
Structural characterisation of powdered solids remains a significant challenge in modern chemistry. Graeme Day, Lyndon Emsley and co-workers have used a combination of computational and solid state NMR approaches to determine the structures of powdered samples of small organic compounds. 
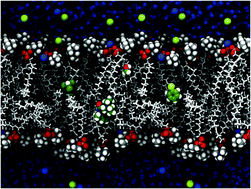 Joakim Jämbeck and Alexander Lyubartsev from Stockholm University describe an effective and novel way to account for atomic polarization in simulations of the partitioning of small molecules between water and lipid bilayers.
Joakim Jämbeck and Alexander Lyubartsev from Stockholm University describe an effective and novel way to account for atomic polarization in simulations of the partitioning of small molecules between water and lipid bilayers.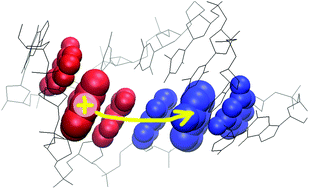 Scientists using computational techniques to look at electron-transfer processes in DNA have employed a surface-hopping approach to predict the degree of charge localisation across nucleobases. The technique should allow more accurate modeling of the effects of charge transfer within the molecule.
Scientists using computational techniques to look at electron-transfer processes in DNA have employed a surface-hopping approach to predict the degree of charge localisation across nucleobases. The technique should allow more accurate modeling of the effects of charge transfer within the molecule.