The outer layer of the skin, the stratum corneum, is a unique barrier membrane. On average it is only 20 μm thick (about a quarter the thickness of a normal sheet of paper) but it prevents us from losing excessive water and it protects us from our environment. It forms a special interface between our body, the air, water and various solids.
In order to understand the barrier properties of the skin we need to determine its structure at various levels ranging from the macroscopic scale to the molecular level. This has been made easier by the advances that there have been over the recent decade. However, the amount of a material that is capable of penetrating this excellent barrier and reaching the underlying systemic circulation is still only of the order of 1 or 2 per cent of the total applied dose.
Read this exciting Perspective article:
Skin: the ultimate interface
Jonathan Hadgraft and Majella E. Lane
Phys. Chem. Chem. Phys., 2011, DOI: 10.1039/C0CP02943B
PCCP special collection
This Perspective is part of a special collection of articles in PCCP on Interfacial processes and mechanisms in celebration of John Albery’s 75th birthday. Watch out for this exciting collection which will be published in a few weeks!











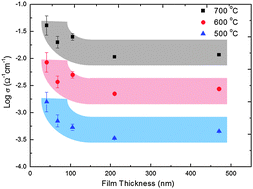 However, especially in the cases of the conductivity of ultra-thin films measured in an in-plane configuration, it is highly possible that the ‘apparent’ conductivity increase originates from electrical current flowing through other conduction paths than the thin film. As a systematic study to interrogate those measurement artifacts, we report various sources of electrical current leaks regarding in-plane conductivity measurements, specifically insulators in the measurement set-up.
However, especially in the cases of the conductivity of ultra-thin films measured in an in-plane configuration, it is highly possible that the ‘apparent’ conductivity increase originates from electrical current flowing through other conduction paths than the thin film. As a systematic study to interrogate those measurement artifacts, we report various sources of electrical current leaks regarding in-plane conductivity measurements, specifically insulators in the measurement set-up.