The fact that modern life relies heavily on fossil fuels is a environmental and a political problem, which will become your problem come when our supplies of fossil fuels run out. Solutions that deal with our need for domestic and industrial energy demands are—more or less—readily available. Our main problem is how to find transportable energy materials to fuel our cars, planes and ships, when no oil-based fuels are available. In the group of Tejs Vegge they have focused on ammonia as a possible mobile energy material.
The main issue in generating next-generation fuels is to remove the energy cost associated with transforming electricity into chemical energy. The solution is catalysts. The manufacture of ammonia, through the Haber–Bosch process, is enabling planet Earth to sustain the 7 billion humans that inhabit it today. If the process were stopped a couple of billion humans would die. Research has made the process of making ammonia very efficient, and has revolutionized our understanding of heterogeneous catalysis. Even so, the Haber–Bosch process is a high-energy process, consuming approximately 2 % of our total energy production.
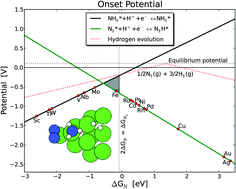
The paper titled ‘DFT based study of transition metal nano-clusters for electrochemical NH3 production’ is focusing on finding a process where electricity can be used to generate ammonia in an electrochemical cell. That is, how to make ammonia efficiently in a small scale, low temperature process on site at/in wind turbines and solar power plants. Using computational chemistry several catalysts are screened.
by Dr Thomas Just Sørensen
If you want to learn more see the paper, which was published in PCCP:
DFT based study of transition metal nano-clusters for electrochemical NH3 production
J. G. Howalt, T. Bligaard, J. Rossmeisl and T. Vegge
Phys. Chem. Chem. Phys., 2013, 15, 7785-7795
DOI: 10.1039/C3CP44641G