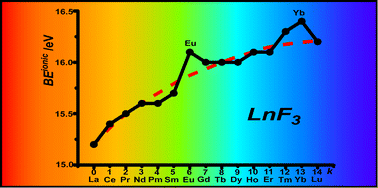
The bonding orbitals in the lanthanide series can, in theory, be 4f, 5d, and 6s orbitals. Traditionally, the contracted f-shell orbitals have been viewed as shielded, and the interaction between lanthanide ions and ions of the opposite charge has to be regarded as purely ionic in nature. This work shows that our current computational models include a significant covalent contribution in the bonding of fluoride to trivalent lanthanide ions.
I look forward to the paper where lanthanide ions with a full coordination sphere are treated, although I fear it will be a long wait. Luckily, the papers reporting the progress towards a full computational treatment of lanthanide in solution are also worth reading, even for experimentalists like me.
On structure and bonding of lanthanoid trifluorides LnF3 (Ln = La to Lu)
Wei Xu, Wen-Xin Ji, Yi-Xiang Qiu, W. H. Eugen Schwarz and Shu-Guang Wang
Phys. Chem. Chem. Phys., 2013, 15, 7839-7847
DOI: 10.1039/C3CP50717C
by Dr Thomas Just Sørensen










